NatureScot Research Report 1211 - Establishing woodland plants in broadleaved woods - interim best practice guidance for conservation translocations
Year of publication: 2021
Authors: Worrell, R., Holl, K., Long, D., Laverack, G., Edwards, C., Fuentes-Montemayor, E. and Crawford, C.L.
Cite as: Worrell, R., Holl, K., Long, D., Laverack, G., Edwards, C., Fuentes-Montemayor, E. and Crawford, C.L. 2021. Establishing woodland plants in broadleaved woods - interim best practice guidance for conservation translocations. Nature Scot Research Report 1211.
This report sets out interim best practice guidance on how to establish small populations of common woodland plants in broadleaved woods that can then spread naturally within the wood over time. This work can be seen in the wider context of conservation translocations in general, although the reintroduction of woodland plant species is typically local scale and non-controversial. The end point should be to develop naturalistic and attractive woodland plant communities which benefit both biodiversity and woodland amenity.
Keywords
woodland plant(s); conservation translocation; reintroduction(s); planting; sowing; seed; best practice guidance
Background
Broadleaved woodland expansion in Great Britain mainly happens by planting trees on former agricultural land. Creation of such new woodland has generally taken place with little or no consideration of outcomes in terms of developing new woodland flora. Broadleaved woodlands established on arable land and improved pasture often develop ground flora communities typical of disturbed land that lack a woodland character, and are dominated by grass and agricultural weeds. These ruderal vegetation communities can be very persistent. Whilst these woods may acquire a few woodland species by natural colonisation, they generally fail to acquire authentic woodland plant communities because the woodland plants are missing from adjacent habitats. One means of addressing this is via targeted conservation translocations of common woodland plants. This report sets out interim best practice guidance on how to establish small populations of common woodland plants that then spread naturally within the wood over time. This work can be seen in the wider context of conservation translocations in general, although the reintroduction of woodland plant species is typically local scale and non-controversial. The end point should be to develop naturalistic and attractive woodland plant communities which benefit both biodiversity and woodland amenity.
Main findings
- Guidance is given on how to foster the development of naturalistic and attractive woodland plant communities in planted broadleaved woodland on agricultural land in situations where this is unlikely to happen naturally.
- The aim is to establish small populations of plants that can act as sources for future natural colonisation within woodlands, rather than attempt to recreate widespread woodland ground cover.
- There are circumstances when large-scale establishment of woodland plants is valid i.e. when new woods are being created in intensively farmed landscapes lacking sources of woodland plants, and where visitor amenity is important from the start.
- Planning starts by considering what improvements to the woodland flora can be made by conventional conservation management – without carrying out conservation translocations e.g. deer control and strengthening local forest habitat networks.
- Where conservation translocations are desirable, the following steps can be taken: a) assess the plant communities present in the woodland; b) assess the species present in adjacent woods and habitats that may colonise naturally; c) assess which plant species would occur naturally in the woodland and d) work out which plant species are missing. These steps comply with the wider context of the ‘Scottish Code for Conservation Translocation’ approach (based on the IUCN Guidelines) which ensures all other important risks/benefits/issues are addressed and in line with the legislation.
- A list of target species most suitable for planting in broadleaved woodlands in Scotland is proposed, comprising 27 broadleaved woodland specialists, 12 woodland-edge species, and 5 grasses. These are common, robust and familiar species that are characteristic of the main types of broadleaved woodland.
- Site treatment prescriptions are given including raking, strimming, treatment of small patches with herbicide and /or cultivation, and using mulches.
- Guidance is given on how to establish plants using seed, plant fragments and potted plants. The best methods to choose depend on the plant species, the budget and scale of operations, how tightly the manager wishes to control outcomes and the condition of the woodland floor.
- It is usually best to collect seed and plants as locally as possible to the planting site. This helps maintain the genetic diversity of woodland plant populations, and helps ensure that plants are well adapted to local growing conditions.
- Good practice in seed collection is outlined and the need for quality seed is stressed. Information is given on planting patterns, timings, costs, records and monitoring.
Acknowledgements
This report is dedicated to the memory of two remarkable women; Joanna Francis for her pioneering work on woodland wildflower conservation translocations and Patsy Wood who loved everything to do with trees and woodlands. The work was funded by Patsy Wood Trust (PWT) and Scottish Forestry (SF) and we are grateful for the solid support from the PWT trustees and Colin Edwards (SF). Many practitioners gave up time to help and we are particularly grateful for input from: Richard Scott (Landlife), Phil Putwain (University of Liverpool); Jill Aitken, Tim Hall, Peter Leeson, Simon Mageean (Woodland Trust), Emilie Wadsworth (Central Scotland Green Network), Kevin Watts (Forest Research) and John Watt. All photographs are the property of Rick Worrell unless otherwise indicated.
Authors
This report has been produced as a collaboration between specialists working within partner organisations, informed additionally by input from a Sharing Good Practice workshop Restoring Plant Communities in our Woodlands – Time for Action on June 8, 2018. The authors and their affiliations are: Richard Worrell (independent forester), Kate Holl (NatureScot, woodland adviser), Deborah Long (Scottish Environment Link), Giles Laverack (Scotia Seeds), Colin Edwards (Scottish Forestry, Environment Policy Adviser), Elisa Fuentes-Montemayor (Scotland’s Rural College (SRUC) and University of Stirling), and Carol Crawford (independent botanist).
Introduction
This report sets out best practice for translocating woodland plants into planted broadleaved woodland in situations where this is unlikely to happen by natural colonisation. Woodlands established on arable land and improved pasture often develop ground flora communities typical of disturbed land that lack a woodland character, and are dominated by grass and agricultural weeds. It should however be noted that some agricultural weeds found in planted woods, such as thistles, buttercups and willowherbs, are often important for pollinators in otherwise floristically poor woodlands. In isolated woods, these vegetation communities can be very persistent. Whilst these woods may acquire a few woodland species by natural colonisation, they generally fail to acquire authentic woodland plant communities. One means of addressing this is via targeted conservation translocations of plants that are missing from the woodland and surrounding areas. A few pioneering practitioners, mainly in England, have carried this out, and this report draws on a review of the evidence from their work. Our current state of knowledge is not sufficient to provide detailed prescriptions with guaranteed outcomes, but the basic approaches and principles are now clear enough that they can underpin broad guidance.
This guidance is potentially applicable across the UK, but the list of species proposed relates to site conditions in Scotland and would need to be adapted for the other countries; and some of the wider government guidance on conservation translocations referred to is specific to Scotland. The legislation that relates to the translocation of species (especially when outwith native range) is well developed in Scotland and a licence may be required. Guidance on conservation translocations in Scotland is available on NatureScot website. Please note that the following types of woodland plant translocations are outwith the scope of this report:
- translocations of specific vulnerable/threatened species
- translocations in the form of ‘assisted colonisation’, to address threats arising from climate change or plant diseases
- translocations into ancient semi-natural woodland sites, or sites with a conservation designation
The guidance contained in this document has been assembled from a range of sources including consolidation of expert knowledge, evidence and experience from around the UK, together with a modest review of the scientific literature to supplement the advice from workshops, reports and toolkits. It has been written as a handbook for woodland owners/land managers and non-specialists that brings together the most up-to-date knowledge around best practice of how to establish a woodland ground flora, and in general, limited expertise should not be a hindrance to its effective use. However, in some parts of the report a more technical discussion has been considered necessary in order to justify/explain the suggested practice. Notwithstanding this, the underlying desire behind writing the report is that it brings together relevant information into guidance, outlining critical considerations and key areas of uncertainty, all of which should be taken into account whilst using a common-sense approach.
All plant names after Stace (2019).
The problem
Creation of broadleaved woodlands in Scotland has generally proceeded with little or no thought of how to develop a natural woodland flora in the long term. When new woodlands are created on mainly semi-natural vegetation in the uplands, leaving the development of woodland vegetation to natural processes is an acceptable position. But as the focus of woodland creation has expanded to include improved pasture and arable land in the lowlands, experience has revealed that outcomes are likely to be poor (see figure 1).
The longer term prospects for these woodlands acquiring woodland ground flora species depend mainly on the proximity of nearby populations of woodland plants. Where the new woodland is adjacent to semi-natural woodland, woodland plant species will gradually colonise the new woodlands over the course of decades or centuries; with the semi-natural woodland acting as a “source”. Some colonisation of more mobile woodland plants may happen in some other circumstances e.g. where the new woodland is part of a forest habitat network or adjacent to semi-natural open habitats. However, where the new woodland is isolated, or in a location where woodland plant species are missing in the wider landscape, very little colonisation will take place. This is particularly true for “woodland specialists” which are known to have poor dispersal capabilities. This has several serious negative consequences for conservation and recreation, which undermine the benefits of tree planting.
Experience of translocating woodland plants
Scientists and managers have now spent 30 years investigating methods of establishing woodland plants in situations where colonisation might not be expected to happen naturally (National Urban Forestry Unit 2000, Francis et al. 1992, Murray et al. 2001, Francis and Morton 2001, Woodland Trust Scotland 2002, Worrell and Francis, 2003, Watts et al. 2016). A variety of approaches have been trialled, ranging from planting small quantities of seed or plants in mature woodland, through to soil inversion and large scale reseeding of land at the time of tree planting. This has shown that establishment of woodland plants in young woodland can markedly improve the ecological condition of the woods compared with areas lacking treatment. Woodland species established successfully into woodlands subsequently spread slowly within those woodlands, and could be expected to colonise in the longer term. Species that have been successfully translocated include: bluebell/ wild hyacinth (Hyacinthoides non-scripta), wood avens (Geum urbanum), primrose (Primula vulgaris), ramsons (Allium ursinum), St John’s worts (Hypericum spp.), greater stitchwort (Stellaria holostea), bugle (Ajuga reptans), opposite-leaved golden saxifrage Chrysosplenium oppositifolium), common dog violet (Viola riviniana), herb robert (Geranium robertianum), yellow pimpernel (Lysimachia nemorum), enchanters nightshade (Circaea lutetiana), greater woodrush (Luzula sylvatica) and wood false brome (Brachypodium sylvaticum). Species such as bluebell/ wild hyacinth (Hyacinthoides non-scripta) and primrose (Primula vulgaris) have spread impressively at some sites from only small original quantities of seed.

Top Left: bare soil under pole-stage ash; Top Right: Sparse grass and moss under mixed woodland; Middle Left: strong grass growth under pole-stage ash; Middle Right: creeping buttercup and rushes under pole-stage ash; Bottom: nettles in young oak woodland.
Establishment of open ground wildflower species in glades and ride-sides within woods can also be successful, is important for pollinators, and adds to the ecological value of the woodland. This report aims to set out best practice for plant conservation translocations based on experience to date.
The following aspects are emphasised:
- it is important to assess whether some plant species might arrive by natural colonisation from nearby habitats, which would render conservation translocation unnecessary
- small scale and low cost approaches are more likely to be attractive to woodland owners
- small numbers of plants can be established which will colonise the woodland naturalistically over long time periods
- genetically appropriate sources of seed and plants should be used
Readers can refer to the Scottish Code for Conservation Translocations to find out more about the process of planning a conservation translocation, taking into account the various risks, benefits and legal issues.
Plant conservation translocation as part of the conservation management of woodland plants
Conservation translocation is only one conservation practice for woodland plants. Different types of woodland have different options for conservation management as follows:
- Ancient semi-natural woodland, core habitat in forest habitat networks, and designated sites. In these high quality woodlands, conservation management focuses on minimum intervention management (mainly deer control); and work aimed at achieving favourable condition. At the landscape scale, these woods act as important sources of woodland species for colonising wider woodland area via habitat networks.
- Majority of broadleaved woodlands, both semi-natural and planted: In these woods natural colonisation by woodland plants is likely to be happening or is anticipated in the future. Their main management needs are deer control, diversifying woodland composition and structure, and improving connectivity.
- Isolated, planted woodland on former improved pasture and arable land: In these woods there is little hope of natural colonisation by woodland plants now or in future due to their isolation. Management should include plant species conservation translocations together with deer control. In the longer term, work to diversify woodland composition and structure, and improving connectivity, is beneficial for plant communities.
This publication deals primarily with category 3 woodlands, though there are circumstances where some types of category 2 woodland would benefit from plant conservation translocation.
Planning conservation translocation of woodland plants
Aims
The aim of plant conservation translocations is to foster the development of naturalistic and attractive plant communities in situations where this is unlikely to happen naturally. The long term outcome should be to re-create ground cover where it is not possible to tell if it had originated naturally or had developed with an element of planting. The aim should usually be to introduce plants that can act as sources for future natural colonisation of the woodland, rather than attempt to recreate complete woodland ground cover. This should allow individual species to occupy the niches best suited to them over an extended time period. This approach also reduces costs, by only having to establish small numbers of plants. However there are circumstances when large-scale planting is valid, particularly when new woods are being created in intensively farmed landscapes lacking sources of woodland plants, and where visitor amenity is important from the start (Woodland Trust Scotland 2002, Murray et al. 2001, Landlife 2006).
A set of principles for guiding woodland plant conservation translocations is set out in Assessing the need for conservation translocations section, and described in more detail in the following sections. These build on the provisions set out in the Scottish Code for Conservation Translocations. Plant reintroductions, or other types of plant conservation translocation defined in the Scottish Code (NSRF 2014), fall under these guidelines.
Management to enhance plant communities - before undertaking any conservation translocations
It is important to consider options for conservation management to enhance plant communities before undertaking any planting, and to try to eliminate, or reduce, any factors that have limited colonisation. The main issues to consider are the need for deer management, strengthening forest habitat networks and in some cases, assisting the spread of existing populations of plants within the wood itself. The NatureScot Woodland Management Manual is a useful reference in relation to understanding how to promote biodiversity in your woodland.
Deer control
Elevated levels of deer grazing can limit the flowering and seeding of woodland plants; and movement of deer into woodlands from surrounding agricultural land can act as a vector for non-woodland plants (i.e. agricultural weeds). It is useful to evaluate the impacts of deer on the woodland, estimate deer populations and put in place a deer management plan. However note that in the long term, low levels of deer browsing has a positive impact on ground flora composition and regeneration establishment, and is a natural part of a woodland ecosystem. Information on deer management is widely available and tools have been developed to support good grazing practice, such as the Woodland Grazing Toolbox.
Strengthening forest habitat networks
A strategic approach is to work towards strengthening the forest habitat network that the wood is located in. This should lead, over long time-scales, to improved opportunities for woodland plants to colonise new woodland naturally. In some woodlands this may be feasible using targeted woodland creation to form links between woodlands. However in agricultural landscapes such an approach is likely to remain more difficult to deliver. An example of this approach is given in Worrell and Long (2010) and further information on forest habitat networks is given Moseley et al. (2008).
Assessing the need for conservation translocations
A considerable proportion of woods do not need plant conservation translocations. These are:
- ancient semi-natural woods: these usually have a good component of woodland plants
- other types of (recent) semi-natural or planted woodlands with reasonably diverse plant communities: these will typically be woods planted or regenerated on semi-natural (unimproved) land such as heathland
- all woods located immediately adjacent to woodland with good semi-natural vegetation communities, from where woodland species may colonise naturally
However some isolated semi-natural woods, especially those in lowland agricultural landscapes that have been heavily grazed, can have poor woodland plant communities, and could be considered for plant conservation translocations.
The sections below outline a 4-step process for determining whether or not there is a need for conservation translocations, and if so, which are the “missing species” that should be established (see figures 2 and 3). This should aim to answer the following questions:
- Which woodland specialist and woodland-edge species are already present in the wood?
- Which species are present in habitats linked to the woodland and therefore might colonise in the longer term?
- Which common species might be expected to occur in the wood, given the site types and woodland types present?
- Which common species are therefore “missing”?
Step 1: Which species are already present in the target woodland?
Plant conservation translocations should not take place in woods that already have appropriate plant communities. So the first step is always to carry out a botanical survey of the target woodland to assess the species present in the different plant communities. This survey should provide lists of woodland and woodland-edge vascular plants. It can be useful to get help from local botanists, especially to cover rarer species and more difficult plant groups such as grasses. The lists could be augmented by a rough assessment of their abundance, say using the DAFOR scale (National Plant Monitoring Scheme 2015).
Step 2: Which plant species are already present in adjacent woodland and habitats?
Lists of species, and information on their abundance and location, should also be produced for any adjacent / linked woodlands, hedgerows or other semi-natural habitats which might act as sources of plants for natural colonisation. Species located here appear to have good prospects for colonisation and do not need to be translocated. Adjacent source woodlands might span a range in terms of their species richness from good to poor, i.e.:
- ancient woodland and old hedges with a good suite of woodland plants including ancient woodland species
- woods and hedges with a more limited range of woodland plants and no ancient woodland species
- woods and hedges with poor suite of woodland plants
Relying on natural colonisation would be the best approach if source woodlands have a good suite of species; in this case it may not be necessary to carry out plant conservation translocations. However if source woodlands have a more limited range of plants and no ancient woodland species, then some species will need to be translocated. If the adjacent woodland has a poor suite of woodland plants, then translocations of multiple species would be necessary (see figure 3).
Principles for woodland plant conservation translocations
Main headings are from the Scottish Code for Conservation Translocations.
Evaluate if conservation translocations are the best option
Principle 1 Suitable woods: Plant translocations should only take place where woods have impoverished plant communities and where missing plant species are unlikely to arrive by natural colonisation.
Principle 2 Plant species for translocation: Only translocate plant species that are missing from the site and unlikely to arrive by natural colonisation; or are clearly under-represented and therefore susceptible to genetic problems. Only translocate relatively common species that would naturally occur in the woodland; focus on robust species that can compete with the existing ground cover, and avoid moving rare species around unless there is a strong conservation case. The conservation translocation of a threatened species is outwith the scope of this report.
Set out desired outcomes and develop a plan
Principle 3 Aims: The aim of plant translocations is to foster the development of naturalistic plant communities in situations where this is unlikely to happen naturally.
Principle 4 Sources for future colonisation: The aim should be to translocate plants that can act as sources of propagules for future natural colonisation of the woodland, rather than attempt to recreate complete woodland ground cover. However there are circumstances when large-scale translocations are valid.
Principle 5 Levels of intervention: Managers should use the lowest level of intervention (e.g. site preparation) necessary to meet objectives.
Principle 6 Timing of conservation translocations: It is usually best to wait until favourable site conditions develop under the woodland canopy, but plants can also be established at time of planting.
Maximise chances of successful establishment of plants
Principle 7 Understand the ecology of the site and plant species: Do not begin with any plant translocations until you have a good understanding of the site conditions of the recipient wood and the ecology of plants being translocated.
Principle 8 Genetics: It is best to collect seed and plant fragments as locally as possible to the planting site. This helps maintain the genetic diversity of woodland plant populations and ensures that plants are well adapted to local growing conditions. When using seed or plants from suppliers, ensure that these are sourced from the same region as the planting locations.
Minimise the risks of harm to biodiversity
Principle 9 Biosecurity: Adopt appropriate animal and plant health quarantine and sanitation procedures to avoid the spread of harmful pests and diseases
Maximise societal benefits
Principle 10 Engagement with relevant/key stakeholders: Identify opportunities for involving local community groups and schools etc., and where appropriate consult with and engage other land-users and stakeholders to identify the potential socio-economic benefits and disbenefits of the conservation translocation(s).
Obtain permissions, adhere to legislation and quality assurance
Principle 11 Legal: All work will need to comply with legal requirements and codes of good practice in terms of adherence to biosecurity procedures; collecting seed and plant fragments, ensuring that the proposed receptor site of a species is not outwith its ‘native range’ in Scotland; and the choice of suitable collection and receptor sites.
Principle 12 Quality assurance: It is important to pay attention to the quality assurance credentials of prospective suppliers; especially that there is evidence that germination has been tested and that the seeds can be traced to their original collection sites.
Record translocations, monitor and communicate outcomes
Principle 13 Monitoring and communication: It is important to keep records of plant introductions, monitor them and communicate the results of projects to others.
Step 3: Which species would naturally occur in the wood?
The next step is to determine which species would naturally occur within the wood (see How to assess which species would occur naturally in a target wood section). This is necessary in order to find out how “botanically impoverished” or otherwise the woodland is.
Working out which species would naturally occur in the woodland or planting site in question requires a good understanding of the site conditions, and their associated plant communities. Different woodland plants tend to be associated with particular soil types and woodland types (see table 2). The best way to do this is to identify which woodland types the target woodland would naturally support - either as the broad habitat types set out in table 2 (oak/birch woodland, ash woodland, alder wet woodland etc.); or better as National Vegetation Classification (NVC) communities (Rodwell ed. 1991, Averis et al. 2004). This then allows you to look up which plant species should be present in those woodlands (see How to assess which species would occur naturally in a target wood section).
It should be noted that people planting trees don’t always get the choice of tree and shrub species right in relation to the soil and climate; so it is important not to simply accept the woodland type based on the planted trees (i.e. oak woodland, ash woodland etc.) at face value.
Step 4: Which species are missing from the woodland and nearby linked habitats?
In order to determine which species are missing and potentially need translocating, the three species lists need to be compared i.e.:
- lists of existing species in the woodland
- lists of species in the surrounding habitats that could colonise
- lists of species usually associated with that type of woodland
This should give a fairly clear idea of which species are missing. Note that if there is any question as to whether a species is native or not to a particular locality, initial checks can be made by referring to the most recent edition of the plant atlas. Translocation of plant species to places outwith their ‘native range’ is not recommended, and it should be noted that, in Scotland any such translocation (which could include places from which it has been locally extirpated) will need a licence from NatureScot (NSRF 2014).
If the woodland/ surrounding habitats have reasonably complete plant communities, then there is no need for translocations. If the woodland and linked habitats have a limited suite of woodland species, then natural colonisation could be relied upon for some species, but the missing species are candidates for translocation. If the woodland and surrounding habitats have only a limited suite of woodland plants then there is a need for translocations.
Note that there can sometimes be a case for reinforcing populations of species, which whilst not missing from the site, are represented by just a few plants. This should be done when there are fears that the population is so small that genetic problems may arise i.e. inbreeding and low genetic diversity.
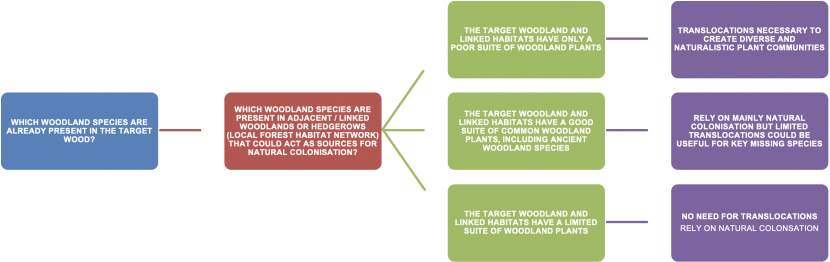
A decision tree to help woodland managers decide whether woodland plant translocations are appropriate for their woodland. Managers are asked to choose three potential outcomes in response to a question about which woodland species are present in adjacent or linked woodlands that could act as sources for colonisation. The first outcome is where the target woodland is well connected to habitats that have a good range of common woodland plants. The advice for this situation is that there is no need for translocations. The second outcome is where the target woodland and linked habitats have a limited suite of plants, and here the advice is that you should wait for natural colonisation, but limited translocation could be useful for key species. The third outcome is where the target woodland and linked habitats have only a poor suite of woodland plants, in which case the advice is that translocations will be necessary to create naturalistic and diverse woodland plant communities.
How to assess which species would occur naturally in a target wood?
There are two main ways to approach this.
Study similar woodlands in the same general area as the target woodland
It is important to study woods in the general vicinity of the target woodland, especially semi-natural woodlands, and understand how woodland plant species change with different site conditions. The plant communities in ancient semi-natural woodland can provide ideas for the mixes of species which might be emulated by translocations (see figure 4). Ideally a range of different woodland types should be visited i.e. oak-birch woodland, ash woodland and alder woodland. This makes it possible to identify and understand the site types and plant communities in the target woodland; which in turns helps identify candidate species for translocation. As a rule of thumb, at least a year should be spent doing this before a list of species to translocate is drawn up.
It is also useful to understand the possible effects of geology and topography, because in some places quite profound changes can happen in relation these factors. For example oak-birch woods on poorer acid sites have several quite different plants associated with them compared to oak-birch woods on fertile lowland sites. Most woods have a mix of site types and woodland types determined primarily by their topography.
The next step is to get familiar with the plant species in table 2, and look for them in the woods in the vicinity of the target woodland. The Forestry Commission bulletin “Creating New Native Woodland” (Rodwell and Paterson 1994) goes a little further, and gives lists of species you might expect to be present for each of the NVC woodland types (as “precursor vegetation” – plant species in open ground prior to tree planting; and “desired invader” - plant species that may colonise woods after establishment). Many of these species might be suitable candidates for translocation.
It is useful to gain a good understanding of the site requirements of individual plant species. One way is to use National Vegetation Community (NVC) Woodland and Scrub (Rodwell et al. 1992). This gives information on which plants might be expected to occur in individual site types within the different NVC communities. The tables for each of the individual woodland types give useful lists of the plant species and indicate their abundance. Although the size and complexity of the book (and its use of only Latin names) can be off-putting, it contains a wealth of information (figure 3).
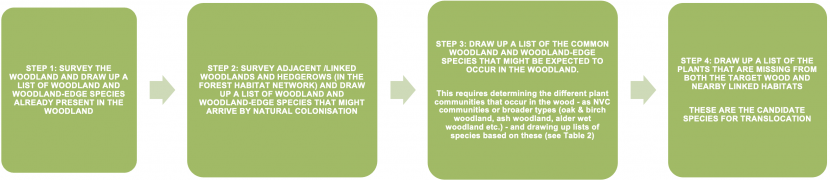
A flow chart that sets out four steps in helping you identify which woodland plant species you might want to translocate. Step 1 asks you to survey the woodland and draw up a list of woodland and woodland edge species already in the woodland. Step 2 asks you to survey adjacent and linked woodland habitats to draw up a list of species that might arrive by natural colonisation. Step 3 requires you to draw up a list of common woodland and woodland edge species that might be expected to occur in the woodland; this requires you to use the NVC classification to identify which woodland communities are present in the wood. Step 4 asks you to use the previous information to identify which plants are missing from the wood and these are the candidate species for translocation.

Study soil conditions
It is useful to gather more detailed information about the soil conditions that individual woodland plant species favour. The soil moisture and nutrient requirements of woodland species determine the range of micro-sites that species prefer. Understanding this makes it easier to identify the best locations within the target woodland to establish individual species; fewer mistakes are made.
One way of doing this is by using Ellenberg values (Hill et al. 1999), which provide indices assigned to individual species describing their preferred soil conditions in terms of soil moisture, soil pH (reaction) and nitrogen requirements. An example of this applied to woodland plants is given in Murray et al. (2001). Alternatively the Forest Research “Ecological Site Classification” can be used, which is rather simpler (Pyatt et al. 2001, see table 1), especially for forest managers already familiar with this system. Soil conditions can usefully be considered as a “grid”, with moisture regime on one axis (dry to wet) and soil nutrient regime on the other (poor to rich). The cells in the grid (see table 1) show the conditions under which species grow best (though in reality species will be found in several cells around the central cell shown).
| - | Soil Nutrient Regime - Very poor | Soil Nutrient Regime - Poor | Soil Nutrient Regime - Moderate | Soil Nutrient Regime - Rich | Soil Nutrient Regime - Very rich |
|---|---|---|---|---|---|
| Soil moisture regime - Slightly dry | - | - | wood sage | - | - |
| Soil moisture regime - Fresh | - | wavy hair grass, bracken, slender St John’s wort | greater stitchwort, broom | bluebell | - |
| Soil moisture regime - Moist | blaeberry, heather | heath bedstraw | wood anemone | tufted hair grass, barren strawberry, herb robert | dog’s mercury, stinging nettle, celandine |
| Soil moisture regime - Very moist | - | soft rush | - | bugle | - |
| Soil moisture regime - Wet | purple moor grass | marsh thistle | yellow pimpernel, creeping buttercup | valerian | - |
| Soil moisture regime - Very wet | - | - | - | opposite-leaved golden saxifrage | - |
Contacting local knowledgeable people can also be useful, e.g. local authority ecologists, community woodland staff, local wildlife trusts, Plantlife, etc.
How to assess the degree of isolation / connectivity of the wood
For planted woodlands that are immediately adjacent to existing woodland, research on plant colonisation shows that over a period of decades, many species will migrate out from the source woodland and start to occupy the new woodland. Migration rates are typically 30-100 cm per year, and can be up to 2 m per year (Brunet 2007, Dzwonko, 2001, Orczewska 2010). In addition, a few longer distance dispersal events are also possible, provided that the seed vectors (e.g. wind, birds, ants) operate adequately. Hence woodland in these situations has the potential to acquire further woodland and woodland-edge species by natural colonisation. However, if the target wood is largely isolated, then the potential for natural colonisation is very low.
The degree of isolation can be assessed by considering habitat networks. For most practical purposes this can be determined simply by inspecting maps, aerial photos and carrying out ground survey to see which woodlands, hedgerows and other semi-natural habitats are linked with the wood in question. Alternatively a more rigorous analysis of the Forest Habitat Network could be carried out using the methodology outlined in Moseley et al. (2008). This takes account of the potential for plants to migrate from source woodlands across intervening habitats.
There is no easy way of assigning woodland to different degrees of isolation. This is because we know so little about the capacity of plants to migrate between woodland areas in agricultural landscapes. In the absence of evidence we can tentatively suggest three categories:
- Woods with good connectivity: Woods with adjacent woodland, hedgerows etc. as part of a forest habitat network. Woods in this situation have considerable potential for colonisation.
- Woods with moderate connectivity: Woods with no adjacent woodland but with connected corridors – hedgerows and/or ditches/watercourses - and with other local woods and semi-natural habitat close by (say within 100m). These have some prospect of colonisation happening over long time periods (many decades or centuries). This might allow more mobile species to colonise within a reasonable time frame, and some less mobile ones might colonise eventually.
- Isolated woods: Woods with no significant woodland or wildlife corridors in the near vicinity: these have very little potential for colonisation, with only the more common and most mobile woodland species likely to colonise in the longer term, and even this is uncertain.
Types of target woodlands and timings of plant translocations
Five main situations can be identified where translocating woodland plants is acceptable and feasible.
Planted woodland after canopy closure
This is the most usual situation and includes all ages of woodland. In the case of older woods (say 30+ years old), there is a good case for going ahead immediately with translocations. For young woods (10 - 30 years) it can be useful to wait and see what happens with the passage of time, then consider the need for translocations. Having said that, for owners keen to progress the colonisation of woodland plants, careful translocations can be beneficial at any stage; this can simply be thought of as reinforcing natural colonisation processes. Note that some heavily stocked, un-thinned woods, especially if they include some sycamore or beech, can be too shady for most plant species to establish and will need thinning (see Competing vegetation on woodland creation sites section).
It is technically feasible to establish plants when the trees are young, but have not closed canopy; but this is the least desirable time point, because weedy vegetation will have become established and it will be several years before this starts to decline due to shading.
Woodland creation sites at time of planting
Establishing woodland plants by seeding at the time of planting is a useful option in sites on highly modified agricultural soils and where persistent competitive ‘weedy’ ground cover is likely to develop. This can be done using the relatively cheap small scale approach set out in this guidance, or by more intensive but expensive methods such as soil inversion and complete re-seeding (Landlife 2006). Species that thrive only in deep shade should probably not be translocated at this stage (see table 2).
Species-poor semi-natural woodland
Birch woodland on former agricultural land, which is isolated from other woodlands, can be a good target for translocations. The vegetation in these woods tends to be dominated by grasses and open-ground species, and lacking in the less mobile woodland species, so can benefit from translocation of woodland plants.
Former conifer clear-fell sites to be planted with native broadleaves
Where native broadleaves are being established following felling of conifers, especially spruce, site conditions in the first and second year after felling often provide excellent conditions for direct seeding of common woodland plants, due to the lack of vegetation and reduced competition.
Former rhododendron sites
Woodlands that have been cleared of rhododendrons are a good target for plant translocations, as seed sources of woodland plants in the immediate vicinity have been suppressed, by the rhododendron ‘canopy’.
Which species to translocate?
A great number of plants have woodland or woodland-edge as their main habitat; for example the National Vegetation Classification lists about 700 species of plant which occur in British woodlands and scrub (Rodwell 1991). However, a much smaller number can be identified which are most suitable for translocating into woodlands, i.e. species that are:
- common and familiar
- locally native
- characteristic of the main types of broadleaved woodland i.e. oak-birch, ash and alder woodland
- known to have limited dispersal capabilities
- quick to establish and grow robustly and are therefore able to overcome competition from other plants
The focus should be on translocating relatively common species – i.e. ones that would certainly occur naturally in the woodland. A list of suitable species commonly found in Scotland for the main broadleaved woodland types is set out in table 2, which is intended as general guidance, rather than a definitive list. This was derived using the criteria above and by consulting lists of species that have been successfully established in research trials (Murray et al. 2001). However, even applying these criteria still leads to subjective outcomes, and different people would come up with slightly different lists.
It is important that the species chosen are known to be locally native at the planting site. This will nearly always be the case for the species in table 2; but if there is any doubt this can be checked using the BRC on-line new atlas. If the ten km square has a blue record (native), then translocation is likely to be supported. If the ten km square is blank or red (non-native assessment) then it is likely to be outwith the native range and translocation is not recommended. However, if following discussion with NatureScot it is agreed that there is a good case for translocation, and the Code is followed, then a licence could be issued. Further guidance on the concept of natural and native ranges are available from NatureScot. If there is any question about whether a species might naturally occur in a wood, or have naturally occurred in recent history, it is best not to introduce it. Managers could also consult local ecologists in order to draw up lists appropriate for individual sites.
A few of the species listed in table 2 are relatively mobile (right hand column) and often colonise woods naturally; so they only need to be translocated where woods are particularly isolated. The woodland specialists page of table 2 lists a few of the more common ancient woodland species, and it is important that some of these are translocated in addition to the more common and mobile woodland edge plants. These are ecologically valuable, highly characteristic of woodland, and least able to colonise naturally.
Managers should note that some woodland species also occur in open habitats such as grassland, heathland and mires (as indicated in table 2), and these species should be encouraged on woodland edges and in open areas within woods.
Herbs, grasses and ferns
Conservation translocations have traditionally focussed on woodland wildflowers (i.e. herbs), which are often the most obvious missing elements. However the same principles potentially apply to some other plant groups, especially grasses. Woodland grasses are an important component of the ground flora; woods missing these clearly benefit from their translocation. An experiment in the US suggested that use of seed mixes with relatively high proportion of grass seed relative to herbs produced just as good outcomes in terms of species richness as expensive mixes with a high proportion of herb seeds (Brudvig et al. 2001).
Not much is known about the need for translocating ferns. Evidence from this project suggests that common woodland ferns appear to be fairly mobile and can colonise from nearby woods. Therefore ferns are omitted from this guidance until more is known about their colonisation capabilities.
Some species of common lower plants i.e. mosses, liverworts and lichens are potentially candidates for translocation; though the task of assessing if these actually require translocation is even more difficult than for vascular plants and requires specialist knowledge. These appear to have similarly variable dispersal capabilities as woodland herbs, varying from highly mobile species to those that are confined to ancient woodlands. It is beyond the scope of this guidance to set out which species might be suitable targets.
| Species - common name | Species - Latin name | Main woodland types that plant species occur in Oak-birch woodland | Main woodland types that plant species occur in Ash woodland | Main woodland types that plant species occur in Alder wet woodland | Broad habitat type - Woodland | Broad habitat type - Woodland edge | Broad habitat type - Open | Shade tolerance from Ellenberg values | Relatively mobile species |
|---|---|---|---|---|---|---|---|---|---|
| Bluebell | Hyacinthoides non-scripta | Yes | Yes | No | Yes | Yes | No | Quite high | No |
| Barren strawberry | Potentilla sterilis | No | Yes | No | Yes | No | No | Quite high | No |
| Bugle | Ajuga reptans | No | Yes | Yes | Yes | Yes | Yes | Quite high | No |
| Cow-wheat, common | Melampyrum pratense | Yes | No | No | Yes | No | No | Quite high | No |
| Dog’s mercury | Mercurialis perennis | No | Yes | No | Yes | No | No | Very high | No |
| Dog violet, common | Viola riviniana | Yes | Yes | No | Yes | Yes | Yes | Moderate | Yes |
| Enchanter’s nightshade | Circaea lutetiana | No | Yes | No | Yes | No | No | High | No |
| Figwort, common | Scrophularia nodosa | No | Yes | No | No | Yes | No | Low | No |
| Golden saxifrage, opposite-leaved | Chrysosplenium oppositifolium | No | No | Yes | Yes | Yes | No | Low | No |
| Greater stitchwort | Stellaria holostea | Yes | Yes | No | Yes | Yes | No | Quite high | No |
| Hedge woundwort | Stachys sylvatica | No | Yes | No | Yes | Yes | No | Moderate | Yes |
| Herb Robert | Geranium robertianum | No | Yes | No | Yes | Yes | No | Quite high | Yes |
| Honeysuckle | Lonicera periclymenum | Yes | Yes | No | Yes | Yes | No | Quite high | No |
| Lesser celandine | Ficaria verna | No | Yes | Yes | Yes | Yes | Yes | High | No |
| Primrose | Primula vulgaris | Yes | Yes | No | Yes | Yes | Yes | Quite high | No |
| Ramsons | Allium ursinum | No | Yes | Yes | Yes | Yes | Yes | Very high | No |
| Red campion | Silene dioica | No | Yes | No | Yes | Yes | Yes | Quite high | Yes |
| St John’s wort, slender | Hypericum pulchrum | Yes | No | No | Yes | Yes | No | Moderate | No |
| Wild strawberry | Fragaria vesca | No | Yes | No | Yes | Yes | Yes | Moderate | No |
| Wood anemone | Anemone nemorosa | Yes | Yes | No | Yes | Yes | Yes | Quite high | No |
| Wood avens | Geum urbanum | No | Yes | No | Yes | Yes | Yes | High | Yes |
| Wood cranesbill | Geranium sylvaticum | No | Yes | No | Yes | Yes | Yes | Moderate | No |
| Wood sage | Teucrium scorodonia | Yes | No | No | Yes | Yes | No | Moderate | No |
| Wood sorrel | Oxalis acetosella | Yes | Yes | No | Yes | Yes | No | Very high | No |
| Wood Speedwell | Veronica montana | Yes | Yes | No | Yes | Yes | No | High | No |
| Woodruff | Galium odoratum | No | Yes | No | Yes | No | No | Very high | No |
| Yellow pimpernel | Lysimachia nemorum | Yes | Yes | Yes | Yes | Yes | No | Very high | No |
| Species - common name | Species - Latin name | Main woodland types that plant species occur in Oak-birch woodland | Main woodland types that plant species occur in Ash woodland | Main woodland types that plant species occur in Alder wet woodland | Broad habitat type - Woodland | Broad habitat type - Woodland edge | Broad habitat type - Open | Shade tolerance from Ellenberg values | Relatively mobile species |
|---|---|---|---|---|---|---|---|---|---|
| Cuckoo flower | Cardamine pratensis | No | No | Yes | No | Yes | Yes | Low | No |
| Devil’s-bit scabious | Succisa pratensis | Yes | Yes | No | No | Yes | Yes | Low | No |
| Bedstraw, heath | Galium saxatile | Yes | No | No | Yes | Yes | Yes | Moderate | No |
| Marsh marigold | Caltha palustris | No | No | Yes | Yes | Yes | Yes | Low | No |
| Meadowsweet | Filipendula ulmaria | No | Yes | Yes | No | Yes | Yes | Low | No |
| Pignut | Conopodium majus | Yes | Yes | No | Yes | Yes | Yes | Moderate | Yes |
| Self heal | Prunella vulgaris | Yes | Yes | No | No | Yes | Yes | Low | No |
| Speedwell germander | Veronica chamaedrys | Yes | Yes | No | Yes | Yes | Yes | Moderate | Yes |
| Tormentil | Potentilla erecta | Yes | No | No | Yes | Yes | Yes | Low | No |
| Vetch, bush | Vicia sepium | No | Yes | No | Yes | Yes | Yes | Moderate | No |
| Water avens | Geum rivale | No | Yes | Yes | No | Yes | Yes | Moderate | No |
| Valerian, common | Valeriana officianalis | No | Yes | Yes | No | Yes | Yes | Moderate | No |
| Species - common name | Species - Latin name | Main woodland types that plant species occur in Oak-birch woodland | Main woodland types that plant species occur in Ash woodland | Main woodland types that plant species occur in Alder wet woodland | Broad habitat type - Woodland | Broad habitat type - Woodland edge | Broad habitat type - Open | Shade tolerance from Ellenberg values | Relatively mobile species |
|---|---|---|---|---|---|---|---|---|---|
| False brome | Brachypodium sylvaticum | No | Yes | Yes | Yes | Yes | No | Very high | No |
| Creeping soft-grass | Holcus mollis | Yes | Yes | No | Yes | Yes | Yes | Moderate | No |
| Sweet vernal-grass | Anthoxanthum odoratum | Yes | Yes | No | Yes | Yes | Yes | Low | No |
| Tufted hair-grass | Deschampsia cespitosa | No | Yes | Yes | Yes | Yes | Yes | Moderate | Yes |
| Wavy hair-grass | Avenella flexuosa | Yes | No | No | Yes | Yes | Yes | Moderate | No |
Shade tolerance
Different species have widely differing light requirements. Some are able to tolerate deep shade and can thrive there because of the reduced competition from other (more light demanding) species. Other species require more light to grow and flower satisfactorily. This means that when planting plants or sowing seed, survival can be enhanced by taking account of the species’ shade tolerance.
Firstly it is useful to distinguish the following broad categories:
- Woodland specialists: these are typically found under conditions of full or partial shade under a woodland canopy e.g. woodruff (Galium odoratum), dog’s mercury (Mercurialis perennis). Woodland specialist plants will actually grow well in open conditions if cultivated in flower beds for example; but in natural habitats they cannot compete with open ground species such as grass, and require the shade from the woodland canopy to shade out these more competitive species.
- Woodland-edge species: these are typically found on the edge of woodland where they abut glades, grassland and heath e.g. red campion (Silene dioica), and self-heal (Prunella vulgaris).
- Plants that appear to be able to thrive in woodland, woodland-edge and open ground e.g. bugle (Ajuga reptans), primrose (Primula vulgaris).
It is important to include all three types of plant in translocation projects, but to plant species only in the shade conditions to which they are suited. An assessment of shade tolerance is given in table 2 using Ellenberg numbers for shade tolerance (Hill et al. 1999). The Ellenberg scores are categorised as shown below:
- very high shade tolerance: capable of growing under very dense shade (Ellenberg scores 3 or less)
- high shade tolerance: capable of growing under full canopy of most trees (Ellenberg score 4)
- quite high shade tolerance: capable of growing under canopy of more light demanding trees and also frequent in canopy gaps (Ellenberg score 5)
- moderate shade tolerance: mainly found in open woodland and canopy gaps (Ellenberg score 6)
- low shade tolerance: usually associated with woodland edges, but sometime found under the canopy, but rarely flowering there (Ellenberg score 7)
It is important to include species that typically occur in open habitats, but are also found in woods, though rarely flowering e.g. meadowsweet (Filipendula ulmaria), and common valerian (Valeriana officianalis). These will flower when the canopy gets disturbed and opened up by wind blow or felling.
Some useful publications list the site characteristics associated with different plant species, the most comprehensive being Grime et al. (1990). To make this easier for non-specialists, some practitioners have condensed lengthy ecological texts (such as Rodwell 1991 and Grime 1990) and produced summaries; for example Highways Agency (2005) and Averis (2013).
Plant succession and invasive species
Translocations might ideally be carried out in a way that mimics natural succession, with more common, robust, pioneer type plants first, followed by later successional species; however, there is no research to date that backs up this approach.
Competitive native species
It is wise to avoid translocating species that can be strongly competitive to the point of being invasive, because these can be hard for other species to dislodge. It is not certain or obvious which species might behave like this particularly with changing climate, but as a precaution the following species may need to be controlled to prevent over-competition: bracken, ivy, bramble, creeping buttercup and ground-ivy. It is worth bearing in mind however, that apart from bracken, these plants are all important for pollinators at different times of the year, although not all will continue to flower under a closed canopy. Sometimes invasive native species arrive naturally, such as rosebay willow-herb, and these need to be monitored and controlled if necessary.
Non-native invasive species
Sometimes people are tempted to introduce non-native species for reasons such as improving bee foraging opportunities and honey production, or simply adding colour. This is not good practice and can easily lead to problems, and in Scotland under the Wildlife and Natural Environment Act (Scotland) 2011 it is illegal to plant any non-native plant in the wild. Sometimes invasive species such as Himalayan balsam and rhododendron arrive naturally as garden escapes, and it is important to eradicate these as soon as possible.
Planting for pollinators
When planning your translocation project remember to include plants that are attractive to a range of pollinators such as bluebell (Hyacinthoides non-scripta), bugle (Ajuga reptans), foxglove (Digitalis purpurea), and primrose (Primula vulgaris), and also many of the flowering plants in table 2.
It is also important to think about the structure of the woodland when planning your planting. For instance, open sunny glades can become sheltered flower-rich habitat enjoyed by many pollinators, and planting flowering shrubs such as hawthorn, dog rose, elder, raspberry, bramble and blackthorn around woodland edges can also provide valuable sources of nectar and pollen for insects. But other aspects of the woodland ground layer can be equally important e.g. areas of dry bare earth for solitary bees and solitary wasps nesting; areas of dense matted grass along fence lines and similar for nesting for bumble bees; as well as undisturbed areas where species can hibernate.
The trees themselves are an incredibly important source of food for bees and other pollinators. Flowering broadleaved trees such as lime and sycamore offer thousands of nectar and pollen-rich flowers in one place early in the season; while hazel, alder and willow are also early sources of nectar and pollen.
Remember to take account of the value to pollinators of any existing flowering plants when assessing woodland plant communities. For example, some agricultural weeds found in planted woods, such as thistles (Cirsium spp.), buttercups (Ranunculus spp.) and willowherbs (Epilobium and Chamaenerion spp.), are often really important for pollinators in otherwise floristically poor woodlands.
The site and how to treat it
Herbivore control
Outcomes will only be good if there are no negative impacts of herbivores (deer, rabbits and livestock) on site likely to prevent successful establishment and spread of ground flora. Deer impacts must be kept at the types of low levels that allow a range of palatable and less palatable species of tree and shrub regeneration to establish (for more information see the Herbivore Impact Assessment method in the Woodland Grazing Toolbox). Whilst some woodland plant species appear to be able to establish themselves in the presence of moderate deer impacts, especially those that spread vegetatively; it is very unlikely that they will flower and seed properly. Where small plots are being established as seed sources, these can be protected temporarily with stock fencing until the plants establish and flower. The capacity of plants to spread within woodlands from these seed sources is largely unknown, but success is likely to be better where deer impacts are lower. Larger areas will require development of a deer management plan that includes setting local cull targets and some consideration of potential herbivore movements from adjacent land. The latter may require site owners to enter into collaborative working relationships with neighbours.
Competing vegetation in woodland
Ground vegetation conditions can be highly variable in woodlands, depending on the factors listed below.
- the age of the wood
- the pre-existing land use
Former arable sites have very variable vegetation communities dependent on the species that happened to colonise after tree planting. Where these plants are shade tolerant and are benefitting from the high nutrient levels, they can remain a problem long after canopy closure. In other cases the weeds reduce in extent following canopy closure. Sites on former improved pasture retain species of grass and herb, especially creeping buttercup which can be quite competitive with some woodland plants.
Young woods are often dominated by aggressive agricultural weeds and grasses. Older woods are generally dominated by grass and a few woodland / woodland-edge species. Some older woods have areas of bare ground or sparse vegetation due to shading that are highly suitable for establishing woodland plants into. Others have more or less complete ground cover. It is important to remember to take account of the value to pollinators of any existing flowering plants when assessing existing plant communities.
The best approach is usually to wait until good site conditions develop under the woodland canopy and then use the least intensive site treatment that delivers the aims of the project. Some types of vegetation cover appear not to hinder the establishment of woodland plants too much, especially if introduced as plants or plant-fragments. Such vegetation swards are termed “open”; in that there appears to be room for translocated species to establish successfully. For example, this appears to be the case with some grass swards comprising species such as wavy hair-grass (Avenella flexuosa), creeping soft-grass (Holcus mollis), Yorkshire-fog (Holcus lanatus), and tufted hair-grass (Deschampsia cespitosa).
Trials suggest that there are generally advantages to carrying out some site treatment, so as to secure better establishment of translocated plants. Sowing seeds directly into established vegetation without any form of site preparation is usually a waste of effort. The type and density of the vegetation sward under closed canopy woodland determines the best options for establishing woodland plants. This can be divided into three broad categories (see figure 5):
- Sites with areas of bare soil and sparse vegetation interspersed with areas of denser vegetation. These are very favourable and treatment of competing vegetation is not required. Plants can be established as seed, plant fragments and potted plants, choosing the most suitable microsites. For example, the pioneering work of Joanna Francis at Milton Keynes was done without site treatment (Francis and Morton 2001). Sites with less than 30% vegetation cover are considered ideal (Highways Agency 2005).
- Sites with continuous cover of non-competitive vegetation such as some species of grass. Plants can be established as plant fragments or potted plants, provided shade levels are suitable. Using seed is feasible for some robust species such as bluebell (Hyacinthoides non-scripta), and red campion (Silene dioica); in general it is best to carry out some site treatment in small patches where seed is to be used.
- Sites with continuous cover of competitive vegetation. Species such as cock’s-foot (Dactylis glomerata), creeping buttercup (Ranunculus repens), ivies (Hedera spp.), docks (Rumex spp.), thistles (Cirsium spp.) and rosebay willow herb (Chamaenerion angustifolium) can be difficult to establish woodland plants into. In this case it is useful to carry out ground treatment at least in the patches where plants are to be established. However take into account the value of these species for pollinators when planning use of herbicides. Vegetation can be treated with herbicide and/ or mulching, and cultivation if necessary. This should be done in large enough patches that these areas are not immediately swamped again by the re-growth of weeds. Herbicide can be used if experience shows that other methods fail, but care should be exercised to avoid drift to non-target species. Plant seed and potted plants / plant-fragments into the prepared patches, focusing on robust competitive woodland species.
Site treatments can be done at any scale, from small plots where the seeds or plants are planted, to strips or larger areas of ground. Obviously the most intensive treatments, such as mulching are only feasible at small scale. In trials, herbicide and cultivation treatments carried out together generally lead to better outcomes.
Competing vegetation on woodland creation sites
When a decision has been taken to establish plants at the time of tree planting, site treatment will almost always be needed, involving cultivation and possibly herbicide treatment (see figure 6). This should only be carried out on sites where the vegetation is dominated by ruderal vegetation, so that there are no conservation losses involved with treatment. There are two aspects to site treatment on agricultural ground:
- efforts to control the competing vegetation
- modification of the topsoil layers to mitigate problems with the weed seed bank, artificially high nutrient levels, compaction and plough pans
Where the previous agricultural use was permanent pasture, the topsoil conditions are typically not so heavily modified that cultivation is needed; but if the sward is dominated by rank grasses and creeping buttercup, cultivation can be useful to free woodland plants from competition for a few years. However leaving some areas with tussocky grass along fences and walls is good for nesting bumble bees, and some bare soil on paths, in glades, and wayleaves for ground nesting solitary bees and wasps. In contrast, on some arable and silage grass, topsoil can be hugely enriched by decades of fertilising and this renders the site highly artificial and hostile to the establishment of wildflowers (Landlife 2006). The options for site treatment at time of tree planting are:
- Herbicide with or without subsequent strimming; the strimming aimed at preventing re-emerging vegetation becoming too competitive or flopping down over the young plants. An example of this is the Woodland Trust trial site at Formonthills, Fife, Scotland (Figure 8) (Woodland Trust Scotland 2002). The aim is to control vegetation, but accept the topsoil conditions that come with the site.
- Cultivation combined with herbicide treatment. The approach pioneered by Landlife and used at several Woodland Trust sites, involves deep ploughing using a Danish plough that effectively buries the modified topsoil and brings subsoil to the surface (Landlife 2006). Herbicide treatment before cultivation eliminates the existing vegetation and makes the cultivation far easier. Herbicide treatment after cultivation is undertaken to control weeds derived from the seedbank, or blowing in from surrounding fields. At a smaller scale, hand cultivation can be used in combination with herbicide.
Shade conditions in woods
Different tree and shrub species cast quite different levels of shade, and so there is a great variability in the light conditions within woods. This will be reflected in the plants that grow under their canopies. Birch and ash woodlands are relatively light canopied; whereas oak casts heavier shade; with alder intermediate. Some shrubs cast heavy shade, e.g. hazel, holly and blackthorn. Non-native tree species such as beech (in Scotland), horse chestnut, and sycamore cast sufficiently heavy shade that they should be avoided for seed sowing sites.
Dense canopy cover has the advantage of shading out unwanted competitive weeds, leaving areas of sparser vegetation and even bare ground, which are potentially suitable for establishing woodland plants. These conditions can arise in the years after canopy closure when the trees are tightly spaced. However, the suitability of woods for establishing plants at this stage is tricky to evaluate because if the shade is dense enough to shade out competing vegetation, it can be too shady for some woodland plants to establish, particularly from seed.
So three aspects need to be considered:
- the level of shade cast by the wood
- the effect this is having on the competing ground vegetation, which depends on the shade tolerance of the species concerned
- the shade tolerance of the plants that are intended to be established
Experience shows that attempting to establish woodland-edge species into conditions of deep shade does not work. Establishing woodland specialists under existing canopy generally works, especially areas of dappled shade with exposed soil (Murray et al. 2001, Highways Agency 2005). There can be advantages to thinning the trees immediately before making the translocations, as this temporarily lets more light in and can give a boost to the newly established plants. If chipping or mulching of thinnings is feasible, the resultant chips left for a year or two can make a good medium for seed germination (Murray et al. 2001). If the existing vegetation is still strongly competitive despite the shade cast by the canopy, herbicide treatment in patches where the plants are to be established can be worth considering.
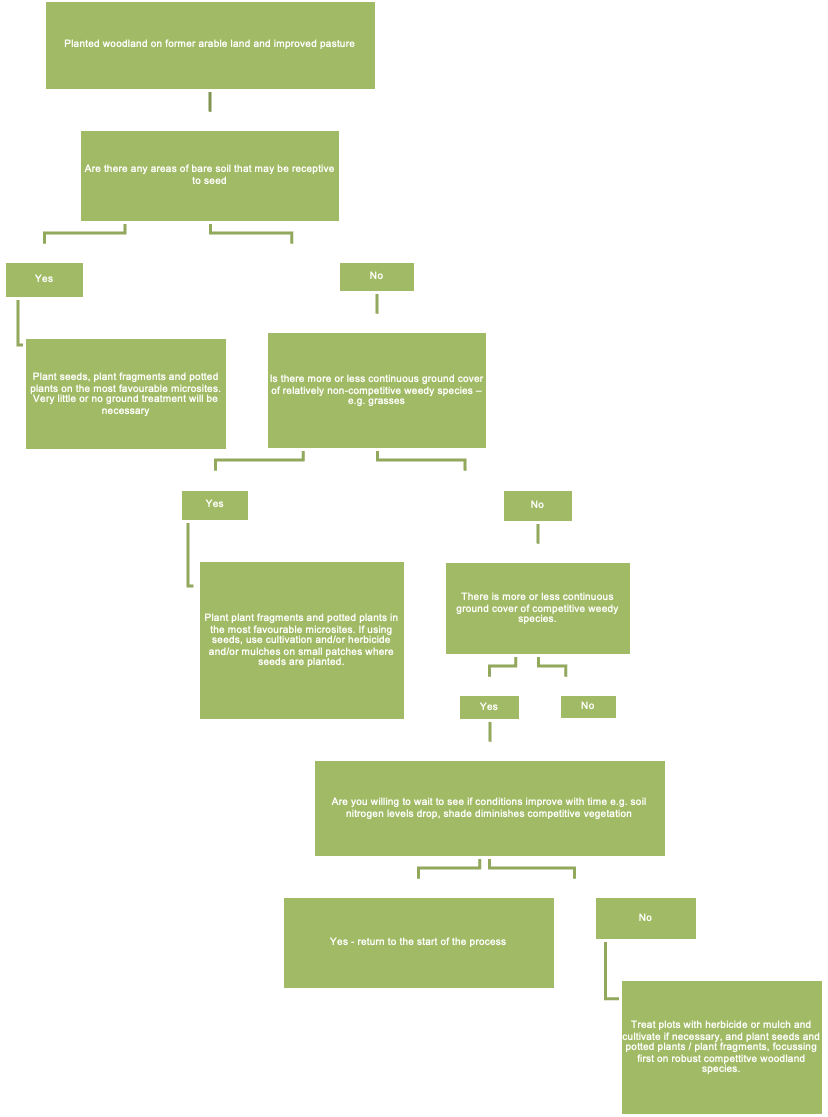
A decision tree to help woodland managers decide the best methods for establishing plants post canopy-closure based on the extent to which the ground is already vegetated. You need to say whether your woodland has areas of bare soil that may be receptive to seed. If the answer is yes, then you can plant seeds or plant fragments etc on favourable microsites with little or no ground treatment. If the answer is no, and there is more or less continuous cover of non-competitive weedy species, then you can plant fragments and potted plants on the most favourable microsites. Cultivation or mulches may be necessary if using seed. If there is more or less continuous ground cover of competitive weedy species then you can either wait to see if conditions improve with time, or treat plots with herbicide or mulch and cultivate if necessary to get translocated plants established.
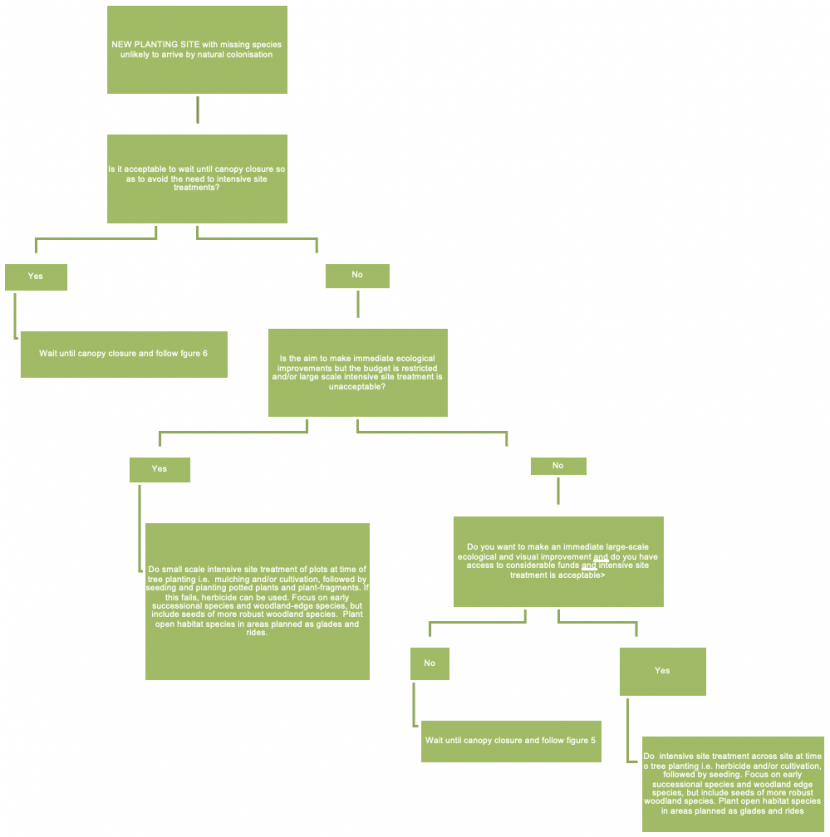
A decision tree to help woodland managers decide the best general approach to plant translocations on a new planting site (or young woodland pre-canopy closure) where missing species are unlikely to arrive by natural colonisation. You need to decide if you are willing to wait until canopy closure to avoid use of intensive site treatments. You then need to decide if you want to make immediate ecological improvements but the budget is restricted and/or large scale intensive site treatment is unacceptable. If this is the case, then you can do small scale intensive site treatment of plots at time of tree planting i.e. mulching and/or cultivation, followed by seeding and planting potted plants and plant-fragments. If this fails, herbicide can be used. Alternatively, if you do want to make an immediate large scale ecological and visual improvement and you do have access to considerable funds and intensive site treatment is acceptable, you can do intensive site treatment across site at time of tree planting i.e. herbicide and/or cultivation, followed by seeding.
Choosing the best sites
The Highways Agency (2005) suggests the best sites can be characterised according to ten criteria including:
- site patchiness i.e. sites with a range of conditions
- light level 15 - 40% daylight at ground level
- existing ground vegetation: less than 30% existing ground vegetation cover
- no dominant weed species (bramble, ivy, bracken)
- leaf litter - at least 30% cover of leaf litter
If a manager is able to select from a number of woodlands, then sites which have the best conditions for establishing woodland plants can be chosen.
How to obtain and establish plants, and Scottish Conservation Translocation Code-related issues
Obtaining seeds and plants
There are several ways of obtaining seed, plant fragments and plants.
1. Collect seed and plant fragments locally
This involves locating suitable wild populations of plants, seeking permission to collect, carrying out collections over the year as seeds mature, and (usually) storing the seed. Typically this would need to happen over more than one year in order to cover a range of species. The seeds and plant fragments can either be planted out directly, or grown on to produce plants. Collecting seed locally adds a considerable time commitment and cost compared with simply buying seeds or plants. It is most suitable for woodland owners willing to devote time themselves or community projects.
2. Contract growing local seeds
Locally collected seed or plant fragments can be passed on to a commercial tree or wildflower nursery to be “contract grown”. Contact needs to be made with suppliers a few years in advance of the intended planting season; together the customer and the business then draw up plans for acquiring and growing seed.
3. Obtaining seed or plants directly from seed suppliers and wildflower nurseries
Seed and plants can be obtained from a seed supplier and/or wildflower nursery. These will typically be from regional populations of stock plants, and woodland managers need to assure themselves that these are genetically suited to the planting site (see Buying seeds section). Check also any plant health considerations under the Scottish Code for Conservation Translocations.
Methods of establishing woodland plants
Woodland plants can be established by:
- Sowing seed in the woodland.
- Planting “plant fragments”, such as divided plants, runners, bulbs and cuttings. These can either be planted directly into the woodland, or grown on in beds or pots and then planted out as rooted plants.
- Potted plants: plants grown in containers such as plugs or pots.
The best methods to choose depend on a number of factors listed below:
The plant species: some species produce large amounts of viable seed that is easy to collect or relatively cheap to purchase, for example red campion (Silene dioica); whilst some others produce little or no viable seed and so are better propagated vegetatively, e.g. wood anemone (Anemone nemorosa).
Risks and control of outcomes: use of potted plants has the best likelihood of successful establishment and leads to the most predictable outcomes. The outcomes of direct seeding are more difficult to predict and partial failures can be expected due to weather (e.g. droughts), or ‘pests’ such as slugs.
Conditions on the woodland floor: if the site is more or less free from competitive vegetation, broadcasting seed and using plants are both good options. On sites with established vegetation using potted plants is usually best.
Budget and scale of operation: direct seeding usually involves the least cost per unit area and is the best approach if the aim is to produce ecological change over a larger area. Use of plant fragments and potted plants has the advantage of creating an immediate impact; but is more expensive per plant and labour intensive. This is usually carried out only at a smaller scale.
Using a combination of direct seeding, together with planting out of plant fragments and plug /potted plants works well. Use seed for those plants that propagate well from seed (see table 3), then other species can be added in using plant fragments and potted plants (see table 4). It is best to allow more than one season to establish plants and progress can be monitored every summer until a good self-sustaining mix of plants is established.
Legal aspects and good practice
Most seed collection is not regulated and people are free to collect seeds of most species from most sites, provided that:
- they have permission from the landowner (without permission there is the prospect that the general law of theft might apply), and
- best practice under the Scottish Code is applied.
There are exceptions and it is important to be aware of these. The Wildlife and Countryside Act 1981 sets out in Section 13 that collection of bluebell (Hyacinthoides non-scripta) seeds for your own use is legal, but a licence from NatureScot is required to sell them.
Collection from protected sites (SSSIs, NNRs) requires permission from NatureScot as well as the landowner. Movement of seeds and plants from one location to another is regulated by the Wildlife and Natural Environment (Scotland) Act 2011 and guidance provided by the Scottish Code for Conservation Translocations and the NatureScot Native Range Guidance.
The following advice for seed collection is adapted from the Millennium Seed Bank Project. Good practice in seed collection includes:
- seeking permission from the landowner and paying attention to Schedule 8 and 13 of the Wildlife and Countryside Act.
- being familiar with collection sites and only collecting from robust plant populations, and only use smaller populations when there is no alternative.
- ensuring that populations are native, not introduced.
- ensuring that collected seed does not constitute more that about 20% of the available seed and ideally a period of a few years should be left between collections.
- recording information on the location, timing and quantities of seed collected.
A useful seed collecting guide, available online as a pdf is the ENSCONET Seed Collecting Manual for Wild Species, produced by the European Native Seed Conservation Network.
Propagation from seed
Seed collection
With a little guidance it is possible to gain the knowledge and skills required to collect seeds. Seeds are living things and need to be treated carefully in order to get the best results. Collecting seeds is time consuming and it can take a long time to gather enough seed to establish a significant number of plants. However, it can be very rewarding and is a great way to involve groups in the process of establishing woodland wildflowers. Some commercial seed producers offer training for community groups and individuals.
Collecting seeds requires knowledge of:
- where there are good populations of particular species
- the timing of flowering and seeding of the species involved
- how to collect and store seeds
- the legal situation and good practice
Information on seed collection for key species is given in table 3. This shows the ease with which seeds can be collected, which varies according to: how abundant the plant is, how much seed it produces and how simple it is to collect. Seed collection is not feasible for some woodland specialists.
Timing
The best time to collect is when the first seeds have just been shed from the plant. Mature seeds are those that are nearest to the positions of the shed seeds and often easily detached, plus they may be dark in colour, and full-looking, hard or dry.
Collecting from robust populations
It is best to collect from large populations of plants which makes collecting fast, has the least impact on the population and helps to ensure a wide genetic base. It is important to try to collect from a reasonable number of unrelated individual parent plants in order to achieve as wide a genetic base as possible; moving through the wood to collect from different populations is good practice. Collection sites are easiest to find and identify when they are growing vigorously or in flower during the early season. By the time the seed is ready to collect there may be no flowers and plants may have died back and be difficult to find or identify, so having a note or even GPS reference of their location is advised. To be effective, collection should focus on places where you can collect from several species at one site. Because the timing of seed production varies between sites, and even between individual plants on one site, collectors should try to monitor the progress of seed maturation, so as to collect at the best possible times.
Collecting and storing
The best ways to collect are shaking or gently picking to ease seeds, or in some cases stripping seeds and seed heads from the plants and putting them in a bag (see table 3). Seeds are best collected into cloth bags which will allow them to start to dry as soon as they are collected. Cotton ‘cash’ bags are good for small amounts of seed, and pillow cases are excellent for larger collections. Strong paper bags which allow seeds to dry are also good if the weather is dry. Plastic bags should be avoided unless there is no alternative and then they should be used for the shortest time possible, as seeds start to deteriorate immediately if kept wet and warm.
As soon as possible, at most within a few hours, seeds should be spread out thinly in an airy dry place. Turn the seeds daily until they seem dry, then leave them a day or two longer before storing in paper or cloth bags. Seeds can be sealed in plastic bags, boxes or jars if it is absolutely clear that they are completely dry. Most species will keep well for a year in dry conditions away from sources of heat. A cool room in a house or, if sealed, a shed is suitable. They should also be kept away from mice, in a box if necessary. Storage in a fridge is not required and can be counterproductive as fridges have high humidity, but if the seeds are in totally airtight containers they can be kept in a fridge. Some seed companies will store valuable collections in their conditioned stores for customers.
Seed dormancy
A dormant seed is one which is alive, but does not immediately germinate when planted, because germination is inhibited by natural features of the seed. Several approaches can be taken to deal with seed dormancy:
- Sow seeds as soon as they are collected without drying them. In some species this prevents the development of dormancy. Bugle (Ajuga reptans) and primrose (Primula vulgaris) are examples.
- ‘Stratify’ seeds using cold. Either sow in autumn so that the low temperatures of winter naturally break dormancy, or place the seeds in damp compost in a fridge for 8 - 10 weeks before planting out. Bugle (Ajuga reptans) and primrose (Primula vulgaris) seeds which have been dried and stored can be treated like this, also bluebell (Hyacinthoides non-scripta).
- Some seed companies can ’prime’ dormant seeds using a range of techniques so that they are ready to grow when they are sown.
Occasionally dormant seeds will take years to germinate either in pots or sown directly in a wood, so it can be worth keeping apparently unsuccessful pot sowings for longer than might be expected.
Buying seeds
It is best to discuss your requirements with a seed supplier and try to get seeds of individual species, or a seed mix that best reflects the site conditions at the planting site. In many cases the standard mixes, which are unlikely to be suitable, can be altered by the seed supplier.
Commercial seed companies offer generic mixes for shady and semi-shade woodland sites which typically contain 15–20 wild flower species alone, or mixed with grasses. Generic woodland wild flower mixes often include species chosen for their colour and reliable germination, and may not have been collected locally (Blakesley and Buckley 2010). They will typically not include woodland specialists; these species are best translocated as plant fragments or potted plants. Most British native seed suppliers are based in southern England and their standard mixes tend to reflect local plant communities. Some of the species they list may include plants which are not found, or are rare, in Scotland and these should not be used. At present the best commercial mixes available in Scotland are those produced in Scotland for "woodland meadows", which would be suitable for planting in more open areas in woods and woodland edges on appropriate sites. To check the native distributions of plants see the online atlas of the British and Irish Flora. Mixes should also contain some appropriate woodland grasses in addition to herbs, but avoid competitive non-woodland grasses such as cocksfoot (Dactylis glomerata).
Quality control of seeds
When buying seeds, it is important that there is evidence that germination has been tested and is satisfactory, and that seeds can be traced to their original collection site.
However, quality control in the wildflower seed trade is undeveloped and the sector is largely unregulated, with the germination of most seed lots being untested. There is evidence that some seed lots being sold have very low germination rates, or are even completely dead; (see Ryan et al. 2008). Seeds of a few species are unlikely ever to be viable, for example wood anemone seeds do not withstand drying so any seeds available for sale are almost certainly dead. Labelling and information about the origin of seeds is often not provided, and seeds are sometimes imported from outwith Britain and Ireland. The use of such seeds or plants in the wild, if these are from distinct populations, might be an offence.
Because of the special status of bluebell (Hyacinthoides non-scripta) in the Wildlife and Countryside Act, native bluebell seeds should only be bought from a wild collection source which has been licensed. Seeds produced from crops grown from wild collections are not subject to licensing but they should be traceable to a native population.
Genetic differences between populations need to be taken into account. Where different subspecies, races or types of species are recognised, a licence is required to translocate these variants outwith their native range (contact NatureScot for more information).
| Species | Ease of collection | Collection time in Scotland | Technique | Tips | Germination, dormancy, sowing time |
|---|---|---|---|---|---|
| Bluebell (Hyacinthoides non-scripta) | Easy | August | Collect seeds when they are black | Visit site when in flower to map out site | Sow immediately in autumn, or store dry and sow in spring. Requires chilling period |
| Barren strawberry (Potentilla sterilis) | Difficult | August | Pick seeds when colour darkens | Few seeds, hard to find | Germinates in warm conditions |
| Common cow-wheat (Melampyrum pratense) | Difficult | - | - | - | A short lived seed and difficult to germinate |
| Common dog violet (Viola riviniana) | Easy | July, Aug | Pick pods when they have fattened and just before they start to open, seeds should be dark, not white | Collect in the early morning before sun and warmth causes pods to shatter | Germinates readily in spring |
| Dog’s mercury (Mercurialis perennis) | Difficult | August | Seed is black and hard when ripe | Very few seeds produced | Strongly dormant and may take years to germinate |
| Enchanter’s nightshade (Circaea lutetiana) | Easy | August | Pick when seeds are dark and losing green colour; they will fall off under light pressure | Can be used as a “hay crop”- simply drop stems with seed at the planting site | Sow in autumn, slow germination |
| Foxglove (Digitalis purpurea) | Easy | July | Seed heads open to reveal ripe seeds | All parts of the plant are poisonous if eaten. Wash hands. Possibly not one for children to collect | Germinates readily in spring sowing |
| Golden saxifrage, Opposite-leaved (Chrysosplenium oppositifolium) | Moderate | July | Grows well from plant fragments | - | - |
| Greater stitchwort (Stellaria holostea) | Moderate | July | Collect seeds when they start to turn brown | Few seeds, shed very readily when ripe | Sow in autumn, needs cold |
| Herb Robert (Geranium robertianum) | Easy | August, Sept | Pick capsules when ripe and showing signs of opening | - | Germinates readily in spring sowing |
| Honeysuckle (Lonicera periclymenum) | Difficult | September | Pick dark berries | Break up berries to pulp and dry | Autumn sowing, germination often poor |
| Lesser celandine (Ficaria verna) | Difficult | June, July | Best done vegetatively using small bulbs | - | - |
| Primrose (Primula vulgaris) | Easy | July, Aug | Seed heads open and dark seeds indicate ripeness | Pick early when slightly green if sowing immediately | Sow immediately or sow in autumn |
| Ramsons (Allium ursinum) | Moderate | August | Pick whole pods. Taking care not to loose ripe seeds. Can also establish plants by dividing tubers | Timing is crucial ; there is only a narrow window when seeds are ripe before they are shed | Sow in autumn, needs cold to germinate. May take two years |
| St John’s wort slender (Hypericum pulchrum) | Easy | September | Seeds dark at ripeness | Shake out seeds into bag or pick whole head | Germinates readily in spring sowing |
| Wild strawberry (Fragaria vesca) | Easy | July | Berries red when seeds ripe | Break berries up to pulp then dry | Germinates readily in spring |
| Wood anemone (Anemone nemorosa) | Moderate | May June | - | Seeds unlikely to succeed | Seed cannot be dried |
| Wood avens (Geum urbanum) | Easy | Aug, Sept | Pick entire seed head when it crumbles under finger pressure. Crumble seed heads into a bag. Both brown and green seeds are viable | - | Seed can be dried and stored and generally germinates well |
| Wood crane’s-bill (Geranium sylvaticum) | Easy | Aug | Pick seed head when they have just opened or are about to open and transfer to a dry box or similar to allow seeds to detach | Seeds turn from green to brown or black when ready to fall. | Germinates readily in spring sowing |
| Wood sage (Teucrium scorodonia) | Easy | September | Seeds turn dark | Strip heads when seeds dark | Sow in autumn |
| Wood-sorrel (Oxalis acetosella) | Difficult | September | - | Very few seeds set | Use plants |
| Woodruff (Galium odoratum) | Difficult | September | Seeds darken when ripe | - | Short lived seed, difficult |
| Yellow pimpernel (Lysimachia nemorum) | Difficult | - | - | Few seeds set | Use plants |
| Bedstraw, hedge (Galium album) | Easy | August | Seeds turn dark | Strip plant when ripe | Germinates readily from spring sowing |
| Devil’s-bit scabious (Succisa pratensis) | Moderate | September | Pick entire seed head a soon as they show signs of starting to break up | Run thumb lightly over seed head – if seeds fall out they are ripe | Slow, sporadic germination in summer |
| Hedge woundwort (Stachys sylvatica) | Easy | September | Pick ripe seed pods when seed are black/brown; or pick entire stalk when mostly ripe and dry out. Seeds ripen sequentially up stem and only a few are released at a time | Can be used as a “hay crop”- simply drop stems with seed at the planting site | Sow in autumn |
| Marsh-marigold (Caltha palustris) | Easy | July | Heads open and seed sits in capsule before dropping | - | Sow immediately when fresh or in autumn |
| Meadowsweet (Filipendula ulmaria) | Easy | September | Pick entire seed heads when they have changed from green to brown | Strip seeds from plant | Slow sporadic germination |
| Pignut (Conopodium majus) | Moderate | July | Pick when seeds brown | - | Sow in autumn, needs cold. May take two years |
| Red campion (Silene dioica) | Easy | July | Collect entire seed heads, or shake seed out of ripe seed heads when seeds have turned black | - | Germinates readily from spring sowing |
| Self-heal (Prunella vulgaris) | Easy | August | Ripe when brown | Pull off whole head | Germinates from spring sowing |
| Tormentil (Potentilla erecta) | Difficult | August- September | Seeds lose green colour | Seeds drop before they look ripe | Germinates readily from spring sowing |
| Vetch bush (Vicia sepium) | Easy | August | Collect pods when they start to turn brown, and store in dry place. Crumble to release from seed from pods | - | Germinates readily in spring |
| Water avens (Geum rivale) | Easy | August | Collect entire seed heads when they change colour from green to brown | - | Sow in autumn |
| Species | Ease of collection | Collection time in Scotland | Technique | Tips | Germination, dormancy, sowing time |
|---|---|---|---|---|---|
| Bedstraw, heath (Galium saxatile) | Easy | August | Seeds turn black | Strip seeds from plant when ripe | Germinates readily from spring sowing |
| Bedstraw, hedge (Galium album) | Easy | August | Seeds turn dark | Strip plant when ripe | Germinates readily from spring sowing |
| Devil’s bit scabious (Succisa pratensis) |
Moderate | September | Pick entire seed head a soon as they show signs of starting to break up | Run thumb lightly over seed head – if seeds fall out they are ripe | Slow, sporadic germination in summer |
| Hedge woundwort (Stachys sylvatica) | Easy | September | Pick ripe seed pods when seed are black/brown; or pick entire stalk when mostly ripe and dry out. Seeds ripen sequentially up stem and only a few are released at a time | Can be used as a “hay crop”- simply drop stems with seed at the planting site | Sow in autumn |
| Marsh-marigold (Caltha palustris) | Easy | July | Heads open and seed sits in capsule before dropping | - | Sow immediately when fresh or in autumn |
| Meadowsweet (Filipendula ulmaria) | Easy | September | Pick entire seed heads when they have changed from green to brown | Strip seeds from plant | Slow sporadic germination |
| Pignut (Conopodium majus) | Moderate | July | Pick when seeds brown | - | Sow in autumn, needs cold. May take two years |
| Red campion (Silene dioica) | Easy | July | Collect entire seed heads, or shake seed out of ripe seed heads when seeds have turned black | - | Germinates readily from spring sowing |
| Self-heal (Prunella vulgaris) | Easy | August | Ripe when brown | Pull off whole head | Germinates from spring sowing |
| Tormentil (Potentilla erecta) | Difficult | August- September | Seeds lose green colour | Seeds drop before they look ripe | Germinates readily from spring sowing |
| Valerian, common (Valeriana officianalis) | Easy | August | Seeds darken and start to drop | Shake into a bag | Sow in autumn |
| Vetch bush (Vicia sepium) | Easy | August | Collect pods when they start to turn brown, and store in dry place. Crumble to release from seed from pods | - | Germinates readily in spring |
| Water avens (Geum rivale) | Easy | August | Collect entire seed heads when they change colour from green to brown | - | Sow in autumn |
| Species | Ease of collection | Collection time in Scotland | Technique | Tips | Germination, dormancy, sowing time |
|---|---|---|---|---|---|
| Creeping soft grass and Yorkshire-fog (Holcus mollis and H. lanatus) | Easy | August | Crush seed with thumb nail – should be hard not milky | - | Easy germination |
| False brome (Brachypodium sylvaticum) | Easy | August | When seeds are hard | - | Easy |
| Sweet vernal-grass (Anthoxanthum odoratum) | Easy | July | Seed turns dark | - | Easy |
| Tufted hair-grass (Deschampsia cespitosa) | Easy | August | Heads open up, seed shakes out | Shake or pick | Easy |
| Wavy hair-grass (Avenellaflexuosa) | Easy | September | Heads open up, seed hard | Shake or pick | Easy |
Propagation from plant fragments
Many woodland plants can be propagated from clumps, runners, bulbs, tubers, and cuttings (see table 4 and figure 7).
Clumps of plants: Many woodland plants grow in clumps e.g. primrose (Primula vulgaris) or mats e.g. wood anemone (Anemone nemorosa), dog’s mercury (Mercurialis perennis), opposite-leaved golden-saxifrage (Chrysosplenium oppositifolium); it does no harm to remove small plants from these. This can be done by cutting round small plants or parts of plants with a knife or trowel, ensuring you get a balanced amount of root and leaf (i.e. not too much leaf for the amount of root).
Runners: Some woodland plants produce runners with developing plantlets on, e.g. bugle (Ajuga reptans), wild strawberry (Fragaria vesca). These can be removed and planted out.
Bulbs, tubers, and root cuttings: Several woodland plants can be grown from bulbs cultivated in a bed, e.g. bluebell (Hyacinthoides non-scripta), bulbils e.g. lesser celandine (Ficaria verna), tubers, e.g. ramsons (Allium ursinum) and root cuttings, e.g. hedge woundwort (Stachys sylvatica).
Cuttings: A few plants can be grown from leaf cuttings, i.e. sections of stem with leaves on, e.g. greater stitchwort, (Stellaria holostea) and yellow pimpernel (Lysimachia nemorum).
The lowest risk approach is to plant out these plant fragments in pots or a nursery bed and grow them on for planting out later when they are more robust. Alternatively they can be transferred directly to the woodland provided the planting spot is reasonably weed free and the soil is moist. This is best done during the growing season in damp weather.
Only ever collect small amounts of plant material from any one source. Only buy bulbs and rhizomes from suppliers with appropriate licences and make sure that this material has been collected legally, and can be planted out legally.
Use of potted plants
Seeds and plant fragments can be grown in pots of compost or soil using standard gardening techniques; or alternatively bought in from nurseries. Pots of various sizes from plugs up to three litres can be used. The larger the pot, the more robust the plant will be at transplanting, and the sooner it is likely to flower and set seed. However larger plants are more expensive. It is beyond the scope of this report to describe methods for raising woodland wildflower plants.
Be careful to avoid garden weed species getting established in the pots, and if purchased from nurseries, consider both genetic issues (see Genetic issues section), and also the potential for introduction of pests and diseases e.g. New Zealand flat worm (see Plant health and biosecurity section).
The range of plants available commercially is limited but includes species such as: bluebell (Hyacinthoides non-scripta), primrose (Primula vulgaris), violets (Viola spp.), and woodruff (Galium odoratum). The seed sources available will typically not be local, so check they may legally be planted out (see Quality control of seeds section).

Top: Plant fragments of yellow pimpernel, bugle and greater stitchwort suitable for direct planting; Middle: grown-on plants of greater stitchwort, bugle and yellow pimpernel suitable for division and planting (NB current recommended practice is to grow plants on raised benches, contact with the ground presents a transmission route for diseases such as Phytophthora); Bottom: recently planted greater stitchwort and wood-sorrel plug plants.

Top: Bluebells from seed (© Woodland Trust Media Library); bugle from cuttings; Middle: primroses and common dog violet from plug plants; opposite-leaved golden-saxifrage and lesser celandine from divided plants; Bottom: primrose from seed (© Woodland Trust Media Library).

Top: in birch woodland and Bottom: on a former spruce restock site.
| Species | Part of plant | Technique |
|---|---|---|
Bluebell (Hyacinthoides non-scripta)
| Bulbs | It is possible to grow bluebells from seed and to dig up bulbs in beds and transplant |
| Bugle (Ajuga reptans) | Runners / cuttings | Cut off sections of runners with emerging roots and plant in damp soil |
Enchanter’s-nightshade (Circaea lutetiana)
| Stolons | Cut off sections of stolons with small plants and plant in damp soil |
| Greater stitchwort (Stellaria holostea) | Cuttings of stem sections | Cut sections of stem during growing season and inserted immediately in damp soil |
Hedge woundwort (Stachys sylvatica)
| Root cuttings | Sections of root can be cut and planted immediately in damp soil |
Lesser celandine (Ficaria verna)
| Small bulbs | Dig up plant and remove small bulbils, with or without stem, and plant |
Pignut (Conopodium majus)
| Tubers | Dig under plants and remove tubers |
Ramsons (Allium ursinum)
| Tubers | Dig up plants and divide tubers |
| Wild strawberry (Fragaria vesca) | Runners | Cut off sections of stolons with emerging roots during growing season and plant in damp soil |
Wood anemone (Anemone nemorosa)
| Stolons | Cut off sections of runners with small plants and plant in damp soil |
| Wood-sorrel (Oxalis acetosella) | Plants | Cut off sections of the matt of plants with small plants and plant in damp soil |
| Yellow pimpernel (Lysimachia nemorum) | Cuttings of stem sections | Cut off sections of runners with emerging roots and plant in damp soil |
Genetic issues
It is usually best to collect seed as locally as possible to the planting site. This helps maintain the genetic diversity of woodland plant populations, and helps ensure that plants are well adapted to local growing conditions (climate, soil conditions, competition with other plants, diseases). At present, this option is only feasible when special seed collections are made, because seed suppliers usually supply from particular populations of stock plants chosen to represent larger regions of the country. Sometimes it is possible to get help with seed collection from seed companies or local conservation organisations.
If locally collected seed is not available, and seed is bought in from a seed supplier, growers should ensure that the source of the seed is in the same general region as the planting site. The effects of transfer of genetic stock from seed source to planting site is an area where research experience and advice is lacking. Perhaps the best practical guidance currently available is that developed for native trees and shrubs (Herbert et al. 1999), which uses a system of “local seed zones”. This suggests a scale of transfer of several tens of kilometres is acceptable, and even more in some circumstances. However there is no evidence that this system is directly relevant for woodland plants, which are generally insect pollinated and patterns of genetic variation may be more fine grained than for (generally wind pollinated) trees.
Some degree of transfer can be thought of as mimicking the large scale geneflow that happens naturally when pollen or seeds happen to move larger distances. The whole issue is complicated by climate change and the increases in introduced plant diseases, which both suggest the need to maintain high levels of genetic diversity. If transfer of seeds and stock is to take place, it is probably best that happens northwards.
The other crucial genetic issue is to ensure sufficient genetic variation in the introduced plants, so that the established population is able to adapt in the future, and does not run into problems with inbreeding depression in later generations because the gene pool introduced was too narrow. Seed should be collected from at least 10 – 20 individuals in the donor plants that are well scattered through the population, not from one particular spot where individuals are likely to be more closely related. For vegetatively propagated plants, it is important that a reasonable number of clones are included in a collection and that this diversity of clones is maintained through the bulking up cuttings. For dioecious plants propagated vegetatively e.g. dog's mercury, is important to ensuring a roughly 1:1 mixture of male and female individuals to maximise seed set.
In the case of bluebell (Hyacinthoides non-scripta), seed collections should only be made from populations where there is no hybridisation with the Spanish bluebell. This is not always easy to establish, so great care should be taken.
Plant health and biosecurity
Conservation translocations present a risk of introducing harmful, exotic pests and pathogens, particularly cryptic soil-borne pathogens such as Phytophthoras.
In addition to the risk of translocation failure, introduced pests and pathogens can infect other woodland plant species. The potential impact to ecosystems and the long-term cost of managing diseases justify investment in good biosecurity practice. More information on plant health considerations for planting schemes is available on Forest Research website.
There are two main routes for the introduction of new pests and pathogens: contaminated plants and soils; and contaminated tools, vehicles and equipment.
To minimise the risk of introducing new pests and pathogens to the woodland, consider the following:
- prioritise propagation by seed and cutting over transplantation of potted plants
- where transplanting is deemed necessary, source plants from local nurseries that can give assurances about biosecurity practices
- protect materials from contamination during transport (use clean containers free of other plant debris)
- ensure that any imported compost, soil, and mulch is of the highest quality, certified and tested for weeds, pests and pathogens
- regularly clean and disinfect tools and equipment to prevent spread of disease
- ensure footwear, vehicle tyres and wheel arches are clean of mud and plant debris when arriving at the woodland
Planting out
Species chosen for planting should reflect the micro-site conditions. Some examples of desirable outcomes are shown in figures 9 and 10. In a woodland with several site types, oakwood species should be planted in areas that are clearly oakwood sites, and ashwood species on ash woodland sites etc. The merging of the two suites of species where the site types intergrade can then occur in the future by natural colonisation. The best approach is to try to emulate patterns seen in nearby similar woods. Planting sites should be conceived of as sources or small centres from which plants can spread. In order to help ensure cross-pollination, it is useful to plant several plants of one species in dispersed groups. Concentrations of plants can be added to over time. There will often be benefits in prioritising planting locations near paths where flowers are most likely to be seen by woodland users.
Numbers of plants and seeds
There is no single best prescription for the numbers of seeds to sow or plants to plant; this depends on the budget for the operation and the degree of impact that managers wish to achieve. The numbers of species being translocated at any time might typically range between 5 and 12 in established woodlands, but could be more in some instances, especially if sowing seed into arable land at time of tree planting. A reasonable minimum number of plants of a specific species might be of the order of 30-60 per species, as this is likely to contain an adequate degree of genetic variation.
Joanna Francis describes several options which keep costs at a reasonable level, but make a noticeable impact (Worrell and Francis 2003) i.e.:
- sow about 10% of the site with a mixture of species at a low sowing rate (say 0.3 g / m2)
- sow a smaller proportion of the area with a mixture of species at a high sowing rate (say 1 – 3 g / m2) – the area sown being dependant on the budget available
- plant patches of plants clustered in groups across the site – numbers again dependant on budget
It is often possible to use areas of established plants within woods as sources for seeds or plant fragments to plant elsewhere in the wood.
Planting out seed
Large quantities of seed can be broadcast by hand (or on large sites being sown at the same time as tree planting, sowing by tractor can be used). Rates of seeding of between 0.1 - 3.0 g/m2 are recommended; with the lower rates for small seeded plants and higher rates for large seeded plants or seed mixtures. Species with high germination rates, which are quick to flower and have high seed numbers are usually woodland-edge species and can be sown at composite rates (in a seed mix) of between 0.1 and 3.0 g/m2. Woodland specialists which have larger seeds, lower germination rates, and are slower to flower should be sown at composite rates of about 3 g/m2. Lower sowing rates can be used on the best micro-sites where germination is likely to be good, and higher ones on poorer sites (Highways Agency 2005).
Seed can be bulked up with a carrier such as sand or fine vermiculite – this allows you to see which areas have been seeded, and so avoid waste. At sowing time seeds of those species suitable for specific parts of the site, can be mixed together. Rake the site prior to seeding to expose as much bare earth as possible, seed into the upper layer of soil and tamp it down or trample it in. On sites liable to drought, it can be useful to top the seeds with a generous layer of sand or grit (Murray et al. 2001). The sower needs to be aware of the site size and layout so that seed is spread evenly over the entire area. Small valuable collections of seed are best sown in trays or pots and grown on for planting out at a later date e.g. primrose (Primula vulgaris) and woodruff (Galium odoratum).
High seedling mortality should be anticipated and only a small proportion of seedlings will survive even on the best sites. On soil that has been cultivated there will be strong competitive growth of seedlings from the seedbank unless these are treated with herbicide or mulch.
“Hay Crops”
A further way of translocating species is via “hay crops”. This involves picking the seeding stalks of plants and laying these out on suitable sites in the target woodland so that the seeds fall directly on the planting site. This can be an advantage for species where it is difficult to extract seed from the seed heads and can help in allowing seeds to mature fully while still on the plant.
Potted plants and plant fragments
These should be planted into micro sites that best suit the individual species. Planting rates of 4 - 9 plants/m2 in small patches are recommended to enhance the likelihood of cross pollination once the plants begin to flower (Francis and Dixie 1996, Worrell and Francis 2003, Highways Agency 2005). It is often more cost effective to use a large number of small plants, than fewer large plants (Highways Agency 2005); but some practitioners consider larger plants to be more robust and therefore “fail safe”; and these can reach flowering age faster. Delivery times of plants should be timed to coincide as closely as possible with planting out, so as to avoid the need for storage. Remove any unwanted weed species that may have rooted in the pots.
Planting times
Planting of both seeds and plants is best carried out in early spring, i.e. March and April. Spring sowing avoids predation over the winter and means that young plants can establish roots and benefit from the entire growing season. However some practitioners, especially in England, prefer autumn or winter (Highways Agency 2005). Potted plants can be successfully established at any time during the growing season, provided dry periods are avoided. It is risky to plant in May because of the likelihood of dry weather. Costs are reduced if seed and plants are planted out at the same time in one operation. Bulbs and rhizomes can be planted at any time between June and October, but ideally before mid-September.
Aftercare
If problems with competitive ground vegetation persist, strimming or weeding targeting specific problem weeds, should be carried out. In the longer term, woodland species often benefit from disturbance to the canopy which lets in enough light to stimulate greater flowering and seeding; and so woodland operations such as thinning and coppicing are beneficial.
Using established plants as seed sources
Once small populations of plants have been established and are flowering and seeding they can be used as seed sources to help colonise the rest of the site. Hopefully this will happen naturally over time. However for owners wishing to accelerate the process these plants can be used as seed sources for further propagation and planting.
Costs
The costs of plant translocations can be broken down as follows (see table 5):
- Site survey to determine need for translocations: to determine the suitability of the site, and which species are candidates for translocations. Some of these items should be covered in standard woodland planning.
- Organising local seed collections: this includes locating suitable sources; obtaining permission to collect; monitoring flowering and seeding and carrying out collections.
- Costs of growing on plants from seed or plant fragments.
- Establishment costs.
- Monitoring.
Planting into woodland
Costs are very variable depending on several factors such as the scale of treatment carried out, and whether seeds or plants are used. Collecting seed / cuttings locally adds considerably to costs compared to obtaining seed from seed suppliers (see table 5). Small-scale planting aimed at simply establishing sources for future natural colonisation is relatively cheap; larger scale translocations aimed at creating a more immediate impact will obviously cost more.
The estimates of cost set out in table 5 suggests that survey work prior to planting might take 2-3 days depending on prior knowledge of the site. Carrying out local seed collections might take about 2-4 person days, depending on the number of plant species involved and the amount of prior knowledge. If plants are used, costs are currently £0.80 - £1.50 per plug plant; and more for larger plants. This translates to £150 - £900 for a programme of establishing 40-60 plants of 4-10 species. Planting costs are probably of the order of 20-40 pence/plant. Planting on this scale might take between about one person day for seeds, or two person-days for plants, plus the cost of any site preparation.
Estimates of seed costs made by Joanna Francis (Worrell and Francis 2003) suggest costs of 1.5-7.5 pence/m2 for freely seeding species up to 30-50 pence/m2 for large seeded species (Prices updated for inflation 2000 - 2015. This would provide more plants per unit area than assumed in table 5.). The costs of a seed mixture is typically 30-50 pence/m2, but varies with the numbers and types of species included.
Planting at time of tree planting
Where woodland plants are established at time of planting using cultivation and weed control, costs will be relatively high. Where Landlife used soil inversion and direct seeding across the entire site, costs were about £1700 per ha (in 2010). However it would be possible to design approaches where translocations were made in patches to act as future sources for colonisation, which would reduce costs considerably.
Societal benefits
The translocation process has the potential to provide social benefits, as the subject is very positive and inspirational. Activities associated with undertaking translocations are very suitable for volunteer involvement (providing health and educational benefits), can strengthen community interactions, and increase the amount of spend in areas local to the donor and release sites. It provides many opportunities for connecting with nature, improving health and well-being, and learning from and about nature.
Practitioners should identify opportunities for involving local community groups and schools etc., and where appropriate consult with and engage other land-users and stakeholders; including identifying the potential socio-economic benefits and dis-benefits. This allows practitioners to plan how they can make the most of any help to do the work on the ground, and also to get people and organisations involved and interested in environmental issues.
| - | Approximate inputs required for a typical 10 hectare wood | Commentary |
|---|---|---|
| 1. Site survey to determine need for translocations | - | - |
| Survey of wood to determine species list of plants present and woodland plant communities | 0.5 – 1 person day | Depends on extent of prior knowledge; and should be included in woodland planning |
| Map based work assessing connectivity / isolation of wood | 0.5 person day | Should be included in standard woodland planning |
| Survey of plant species in adjacent or nearby woodland, hedges etc. | 0.5 person day | - |
| Related reporting, maps, species lists | 0.5-1 person day | Partly included in standard woodland planning |
| Total - site survey | 2-3 depending on prior knowledge
| - |
| 2. Organising local seed collections | Approximate inputs required for 4-10 species, collected from 1-2 nearby woods | - |
| Locating suitable source woods and seed sources within those woods and seeking permissions | 0.5 – 1 person day | Depends on extent of prior local knowledge |
| Monitoring flowering /seeding | 0.5 – 1 person day | Say 2-3 two-hour trips, |
| Collecting seed and plant fragments, seed storage | 1 - 2 person days | Depends partly on travel time |
| Total - collection | 2 – 4 person days | Depends on extent of prior local knowledge |
| 3. Costs of growing on plants from seed or plant fragments | Costs (£) of producing / buying 40-60 plug plants per species | - |
| Per species | £40- 90 per species | - |
| Total for 4-10 species | £160- 900 | - |
| 4. Establishment costs | Approximate inputs required to establish 4-10 species | - |
| Site preparation | 0.5 person day | |
| If sowing seeds (4-10 species) to establish 30-50 plants per species | 1 person day | Plus travel time |
| If using plants / plant fragments (30-50 plants per species) | 1 - 2 person day | Plus travel time |
| Total establishment 30-50 plants of 4-10 species | 2 – 3 person days | Plus travel time |
| 5. Monitoring | - | - |
| Monitoring outcomes | 0.5 days per year | - |
Monitoring and records
It is important to keep records of plant translocations. This serves three purposes, i.e. to:
- inform people which species were planted, to enable them to better understand the ecological developments in the wood
- document the outcomes of different establishment treatments
- understand the future distributions of plants
Records should include: the species planted, the sources of the seeds and plants used, the timing of operations, the treatments used and the outcomes. Note that NatureScot’s Translocation Project Form is a good format for recording how the work was carried out, and can be found in Appendix 1 of The Scottish Code for Conservation Translocations. Ideally records of translocations should be lodged with the Botanical Society of Britain and Ireland.
Monitoring of outcomes should be done twice annually to begin with, and then at longer intervals. Usually a “diary style” is adequate, but ideally more detailed information on ecological change should be gathered using permanent plots and fixed point photography.
References
Averis, A., Averis, B., Birks, J., Horsfield, D., Thompson, D. and Yeo, M. 2004. An Illustrated Guide to British Upland Vegetation, JNCC, Peterborough.
Averis, B. 2013. Plants and Habitats: An Introduction to Common Plants and Their Habitats in Britain and Ireland. ISBN 978-0-9576081-0-8.
Blakesley, D. and Buckley, P. 2010. Managing your woodland for wildlife.
Brudvig, L.A., Mabry, C.M. and Mottl, L.M. 2001. Dispersal, not understorey light competition, limits restoration of Iowa woodland understory herbs. Restoration Ecology, 19, 101, 24-31.
Brunet, J. 2007. Plant colonization in heterogeneous landscapes: an 80-year perspective on restoration of broadleaved forest vegetation. Journal of Applied Ecology 44, 563- 572.
Crawford, C.L. 2009. Ancient woodland plant indicator species in Scotland. Scottish Forestry, 63(1), 6-19.
Dzwonko, Z. 2001. Migration of vascular plant species to a recent wood adjoining ancient woodland. Acta Societatis Botanicorum Poloniae 1, 71- 77.
Francis, J. and Dixie, G. 1996. Planting mixes based on the National Vegetation Classification System. Blandford Forum: H.V. Horticulture Ltd., Dorset.
Francis, J.L., Morton A.J. and Boorman, L.A.1992.The establishment of ground flora species in recently planted woodland. Aspects of Biology 29, 171-178.
Francis, J.L. and Morton, A.J. 2001. Enhancement of amenity woodland field layers in Milton Keynes. British Wildlife, 12(4), 244-251.
Grime, J.P., Hodgson, J.G. and Hunt, R. 1990. The Abridged Comparative Plant Ecology. Chapman and Hall, London.
Herbert, R., Samuel, S. and Patterson, G. 1999. Using local stock for planting native trees and shrubs. Forestry Commission Practice Note 8. Forestry Commission, Edinburgh.
Highways Agency, 2005. The establishment of an herbaceous plant layer in roadside woodland. Design manual for roads and bridges. Vol 10 Section 3. Highways Agency.
Hill, M.O. Mountford, J.O., Roy, D.B. and Bunce, R.G.H. 1999. Ellenberg’s indicator values for British plants. ECOFACT Vol. 2. DETR.
Landlife, 2006. Topsoil Inversion: breaking new ground in forestry. Landlife, Liverpool.
Moseley, D.G., Ray, D., Humphrey, J.W. and Watts, K. 2008. Forest Habitat Networks Scotland. Report to Forestry Commission Scotland and Scottish Natural Heritage.
Murray, M., Green, P., Luscombe, G. and Scott, R. 2001. Woodland Wildflowers Work. Landlife, Liverpool.
National Plant Monitoring Scheme, 2015. Survey Guidance Notes.
National Species Reintroduction Forum, 2014. The Scottish Code for Conservation Translocations. Scottish Natural Heritage, Inverness.
National Urban Forestry Unit, 2000. Urban Forestry in Practice: Establishing wild flowers in recently planted woodland. Wolverhampton.
Orczewska, A. 2010. Colonization capacity of herb woodland species in fertile, recent alder woods adjacent to ancient forest sites. Polish Journal of Ecology, 58, 297-310.
Peterken, G.F. 1981. Woodland Conservation and Management. Chapman and Hall, London.
Pyatt, G., Rasy, D. and Fletcher, J. 2001. An ecological site classification for Great Britain. Forestry Commission Bulletin 124. Forestry Commission, Edinburgh.
Rodwell, J.S. 1991. British Plant Communities. Vol. 1 Woodland and scrub. Cambridge University Press, Cambridge.
Rodwell, J. and Patterson, G. 1994. Creating New Native Woodlands. Forestry Commission Bulletin 112. Forestry Commission, Edinburgh.
Ryan, N., Laverack, G. and Powell, A.A. 2008. Establishing quality control in UK wildflower seed production. Seed Testing International, 135, 49-53.
Scottish Forestry, n.d. Woodland Grazing Toolbox.
Stace, C. 2019. New Flora of the British Isles. Fourth Edition. CandM Floristics, Suffolk.
Scottish Natural Heritage, 2014. Native Range Guidance.
Scottish Natural Heritage, 2018. Woodland Management Manual.
Watts, K., Fuentes‐Montemayor, E., Macgregor, N.A., Peredo‐Alvarez, V., Ferryman, M., Bellamy, C., Brown, N. and Park, K.J. 2016. Using historical woodland creation to construct a long‐term, large‐scale natural experiment: the WrEN project. Ecology and Evolution, 6(9), 3012-3025.
Woodland Trust Scotland, 2002. The management of flora in new woodlands. Seminar Proceedings, Glenrothes, Scotland. Woodland Trust Scotland.
Worrell, R. and Francis, J. 2003. Establishing woodland flora in broadleaved and conifer woodlands. Scottish Forestry, 57, 4, 203-210.
Worrell, R. and Long, D. 2010. Management of Woodland Plants in Atlantic Broadleaved Woodland – A Conservation Framework. Plantlife Scotland, Stirling.
Annex 1
The Scottish Code on Conservation Translocations
Evaluate whether a conservation translocation is the best option
- Undertake an assessment of whether other management actions may be more appropriate (or complementary) in providing a lower-risk, lower-cost, less interventionist conservation solution
Where translocation is the best option, develop a plan to deliver a defined conservation benefit
- Establish the desired outcome: this should be to improve the conservation status of the focal species/habitat by enabling more individuals/populations to survive in the wild; and also to provide wider benefits to biodiversity and people
- Develop a plan including goals and actions, assessment of feasibility and desirability, risk and resource needs, monitoring and management actions (including integration with other conservation actions), and an exit strategy; the depth of planning should be proportionate to the level of risk
Stay legal: obtain necessary permissions and adhere to relevant legislation
- Obtain permissions from landowners before collecting or releasing organisms in the wild
- Consult with Scottish Natural Heritage before undertaking translocations which involve protected species or designated sites, or moving species outwith their native range; obtain all necessary legal permissions and licences
- Where the translocation involves moving organisms to/from other countries, obtain all necessary import/export permissions and licences, and consult with the relevant statutory bodies in all involved countries to establish national legislative requirements
- Adhere to any relevant welfare, health and safety, biosecurity, quarantine and sanitation legislation
Maximise chances of successful establishment of the translocated population
- All translocations must be grounded in a thorough knowledge of the species’ ecological requirements
- Avoid selecting donor populations that have reduced genetic diversity or are likely to be poorly adapted to the release site
- Ensure that the release site and wider area meets all necessary requirements for survival and maintenance of healthy populations into the foreseeable future
- Select the timing, life stage and numbers/sexes of individuals to be released based on the reproductive ecology of the focal species and likely seasonal changes in survival/establishment
- Deliver ongoing management to help translocated individuals survive and become established
Minimise the risks of harm to biodiversity
- Do not remove organisms from a donor site if it will place that population at risk
- Adopt high standards of welfare, and adopt strategies to avoid stress, harm or mortality during the translocation and subsequent release and monitoring
- Adopt appropriate animal and plant health quarantine and sanitation procedures to avoid the spread of harmful pests and diseases
- Evaluate whether establishment at the release site is likely to lead to unacceptable, negative effects on species, habitats or the wider ecosystem, and do not proceed if this is likely to occur
- Evaluate the likelihood of the species or its genes becoming problematically invasive following the translocation, and do not proceed if this is likely to occur
- Avoid mixing highly divergent populations that are likely to be genetically incompatible
- Take particular care where translocations involve islands or isolated water bodies to avoid disrupting their natural isolation from invasive species, pests and diseases
- Translocation of species into areas where they have not previously occurred naturally should only be undertaken if the desired conservation outcome cannot be achieved by other means
Maximise societal benefits and minimise conflict with other land-users
- Consult with other land-users and stakeholders to fully understand the potential socioeconomic consequences of conservation translocations as part of the process of deciding whether it is acceptable to proceed, noting that the benefits and costs of a conservation translocation may be unequally distributed among different stakeholders/land-users
- Evaluate the potential for a translocation to lead to economic or cultural benefits, and identify how any benefits can be targeted
- Evaluate the potential for a translocation to cause harm to human health, well-being and livelihoods and only proceed if acceptable mitigation and management mechanisms can be identified and delivered appropriately
- Have resources in place, and clarity on financial liabilities and legislative restrictions, to deliver any necessary ongoing management and, in exceptional circumstances, to enable reversal of a translocation should unforeseen and unacceptable consequences arise
Record translocations and monitor, evaluate and communicate outcomes
- Monitor translocations to evaluate success and to inform any necessary ongoing management interventions
- Document the translocation and share findings to inform future strategies and projects
Annex 2
Species found in broadleaved woodland and woodland-edge habitats, showing those listed as ancient woodland indicators; those which are considered to be slow or fast colonisers; and those which adapted to flourish in open habitats as well as woodlands. Sources: Peterken (1981) and Crawford (2009).
| Species - common name | Species - Latin name | Ancient Woodland Indicator species | Slow / Fast Coloniser | Occurs in open habitats as well as woodland |
|---|---|---|---|---|
| Angelica, wild | Angelica sylvestris | - | - | Yes |
| Bedstraw, heath | Galium saxatile | - | - | Yes |
| Bedstraw, common marsh | Galium palustre | - | - | Yes |
| Bellflower, giant | Campanula latifolia | Ancient | - | - |
| Bellflower, nettle-leaved | Campanula trachelium | - | Slow | - |
| Betony | Betonica officianalis | - | - | - |
| Bilberry (Blaeberry) | Vaccinium myrtillus | - | - | Yes |
| Bluebell | Hyacinthoides non-scripta | Ancient | - | - |
| Bracken | Pteridium aquilinum | - | - | Yes |
| Bramble | Rubus fructicosus | - | Fast | Yes |
| Bugle | Ajuga reptans | - | - | Yes |
| Buttercup, creeping | Ranunculus repens | - | - | Yes |
| Buttercup, goldilocks | Ranunculus auricomus | Ancient | - | Yes |
| Campion, red | Silene dioica | - | Fast | - |
| Celandine, lesser | Ficaria verna | - | - | Yes |
| Cowslip | Primula veris | - | - | Yes |
| Cow-wheat, common | Melampyrum pratense | Ancient | Slow | Yes |
| Creeping lady’s tresses | Goodyera repens | Ancient | - | - |
| Devil’s-bit scabious | Succisa pratensis | - | - | Yes |
| Dog’s mercury | Mercurialis perennis | Ancient | - | - |
| Enchanter’s nightshade | Circaea lutetiana | Ancient | Fast | - |
| Figwort, common | Scrophularia nodosa | - | Slow | Yes |
| Forget-me-not, wood | Myosotis sylvatica | Ancient | - | Yes |
| Foxglove | Digitalis purpurea | - | - | - |
| Golden-saxifrage, opposite-leaved | Chrysosplenium oppositifolium | Ancient | - | Yes |
| Ground-ivy | Glechoma hederacea | - | Fast | - |
| Guelder-rose | Viburnum opulus | Ancient | - | - |
| Hedge woundwort | Stachys sylvatica | - | Fast | - |
| Hemp-agrimony | Eupatorium cannabinum | - | - | Yes |
| Herb-robert | Geranium robertianum | - | Fast | - |
| Honeysuckle | Lonicera periclymenum | - | Fast | - |
| Horsetail, wood | Equisetum sylvaticum | Ancient | Slow | - |
| Ivy, common | Hedera helix | - | Fast | - |
| Lily-of-the-valley | Convallaria majalis | - | Slow | - |
| Marsh-marigold | Caltha palustris | - | - | Yes |
| Meadowsweet | Filipendula ulmaria | - | - | Yes |
| Moschatel | Adoxa moschatellina | Ancient | - | - |
| Bird’s-nest orchid | Neotia nidus-avis | Ancient | Slow | |
| Orchid, common spotted | Dactylorhiza fuchsii | - | Fast | Yes |
| Orchid, early-purple | Orchis mascula | - | Slow | - |
| Pignut | Conopodium majus | Ancient | - | Yes |
| Primrose | Primula vulgaris | Ancient | Slow | Yes |
| Ramsons (wild garlic) | Allium ursinum | Ancient | - | - |
| Raspberry | Rubus idaeus | - | Fast | - |
| Sanicle | Sanicula europaea | Ancient | Fast | - |
| Self-heal | Prunella vulgaris | - | - | Yes |
| Speedwell, germander | Veronica chamaedrys | - | - | Yes |
| Speedwell, wood | Veronica montana | A Ancient | - | - |
| Stinging nettle | Urtica dioica | - | Fast | |
| Stitchwort, greater | Stellaria holostea | Ancient | - | Yes |
| Stitchwort, wood | Stellaria nemorum | Ancient | - | - |
| St John’s-wort, hairy | Hypericum hirsutum | - | - | - |
| St John’s-wort, slender | Hypericum pulchrum | Ancient | - | Yes |
| Strawberry, barren | Potentilla sterilis | Ancient | Slow | - |
| Strawberry, wild | Fragaria vesca | Ancient | - | Yes |
| Thistle, marsh | Cirsium palustre | - | - | Yes |
| Toothwort | Lathraea squamaria | Ancient | Slow | - |
| Tormentil | Potentilla erecta | - | - | Yes |
| Twayblade, common | Listera ovata | - | Fast | - |
| Valerian, common | Valeriana officinalis | Ancient | - | Yes |
| Vetch, wood | Vicia sylvatica | Ancient | Slow | - |
| Vetch, bush | Vicia sepium | - | - | - |
| Vetchling, bitter | Lathyrus montanus | - | Slow | - |
| Vetchling, meadow | Lathyrus pratensis | - | - | Yes |
| Violet, dog | Viola riviniana | - | Fast | Yes |
| Water avens | Geum rivale | - | - | - |
| Wintergreen, chickweed | Lysimachia europaea | Ancient | - | Yes |
| Wood anemone | Anemone nemorosa | Ancient | Slow | Yes |
| Wood avens | Geum urbanum | - | Fast | - |
| Wood melick | Melica uniflora | Ancient | - | - |
| Woodruff | Galium odoratum | Ancient | Slow | - |
| Wood sage | Teucrium scorodonia | - | - | Yes |
| Wood-sorrel | Oxalis acetosella | (Ancient) | - | Yes |
| Yellow pimpernel | Lysmachia nemorum | Ancient | Slow | - |
| Species - common name | Species - Latin name | Ancient Woodland Indicator species | Slow / Fast Coloniser | Occurs in open habitats as well as woodland |
|---|---|---|---|---|
| Creeping soft-grass | Holcus mollis | - | - | Yes |
| False brome | Brachypodium sylvaticum | Ancient | Fast | - |
| Wood fescue | Festuca gigantea | Ancient | Fast | - |
| Rough meadow grass | Poa trivialis | - | Fast | - |
| Purple moor grass | Molinia caerulea | - | - | Yes |
| Sweet vernal-grass | Antoxanthum odoratum | - | - | Yes |
| Tufted hair-grass | Deschampsia caespitosa | - | - | Yes |
| Wavy hair-grass | Avenella flexuosa | - | - | Yes |
| Wood millet | Milium effusum | Ancient | - | - |
| Woodrush, greater | Luzula sylvatica | Ancient | Slow | Yes |
| Species - common name | Species - Latin name | Ancient Woodland Indicator species | Slow / Fast Coloniser | Occurs in open habitats as well as woodland |
|---|---|---|---|---|
| Beech fern | Phegopteris connectilis | Ancient | - | - |
| Broad buckler fern | Dryopteris dilitata | - | Fast | - |
| Male fern | Dryopteris felix-mas | - | Fast | - |
| Oak fern | Gymnocarpium dryopteris | Ancient | - | - |






